Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The hydrogen spectrum is complex. When a hydrogen atom absorbs a photon, it causes the electron to experience a transition to a higher energy level.
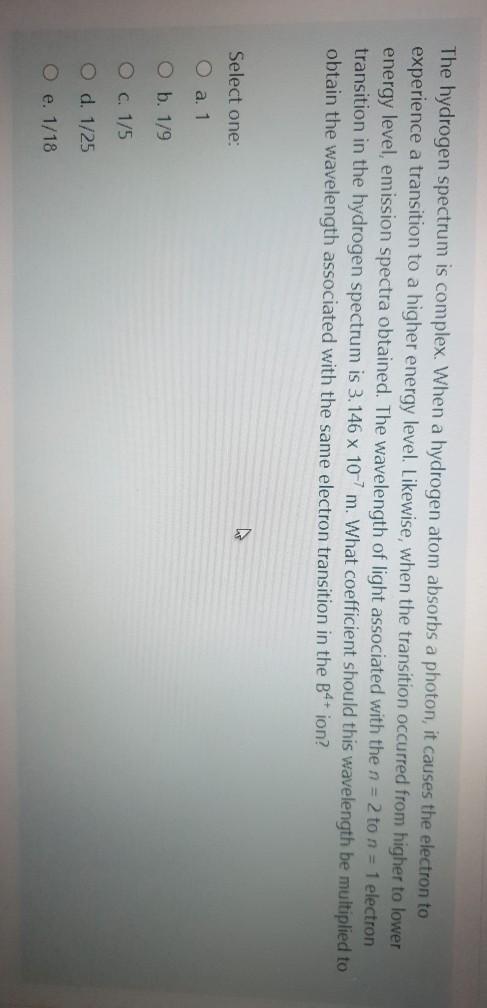
The hydrogen spectrum is complex. When a hydrogen atom absorbs a photon, it causes the electron to experience a transition to a higher energy level. Likewise, when the transition occurred from higher to lower energy level, emission spectra obtained. The wavelength of light associated with the n = 2 to n = 1 electron transition in the hydrogen spectrum is 3.146 x 10-7 m. What coefficient should this wavelength be multiplied to obtain the wavelength associated with the same electron transition in the B4+ ion? Select one: O a. 1 O b. 1/9 O c. 1/5 O d. 1/25 O e. 1/18
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


