Question
The ideal gas model serves as a basis for computing reliable estimates of P-v-T behavior of real gases. A) Briefly explain the conditions under
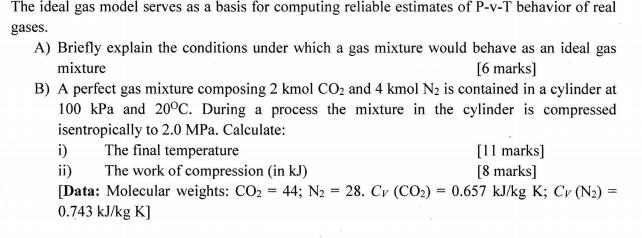
The ideal gas model serves as a basis for computing reliable estimates of P-v-T behavior of real gases. A) Briefly explain the conditions under which a gas mixture would behave as an ideal gas mixture [6 marks] B) A perfect gas mixture composing 2 kmol CO2 and 4 kmol N2 is contained in a cylinder at 100 kPa and 20C. During a process the mixture in the cylinder is compressed isentropically to 2.0 MPa. Calculate: i) ii) [Data: Molecular weights: CO2 = 44; N2 = 28. Cv (CO2) = 0.657 kJ/kg K; Cv (N2) 0.743 kJ/kg K] [11 marks] [8 marks] The final temperature The work of compression (in kJ) %3D
Step by Step Solution
3.32 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
International Financial Management
Authors: Cheol S. Eun, Bruce G.Resnick
6th Edition
71316973, 978-0071316972, 78034655, 978-0078034657
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



