Question
The integrated rate law allows chemists to predict the reactant concentration after a certain amount of time, or the time it would take for a
The integrated rate law allows chemists to predict the reactant concentration after a certain amount of time, or the time it would take for a certain concentration to be reached. The integrated rate law for a first-order reaction is:
![]()
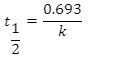
This equation calculates the time required for the reactant concentration to drop to half its initial value. In other words, it calculates the half-life.
Half-life equation for first-order reactions: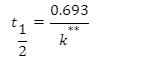
where t1/2 is the half-life in seconds (s), and k is the rate constant in invers (s-1).
Part A
To calculate the half-life, plug the value for k into the half-life equation a What is the half-life of a first-order reaction with a rate constant of 8.60 Express answer with the appropriate units.
[A] = [A]e -kt
Step by Step Solution
3.57 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


