Question
The integrated rate laws for zero-, first-, and second- order reaction may be arranged such that they resemble the equation for a straight line,
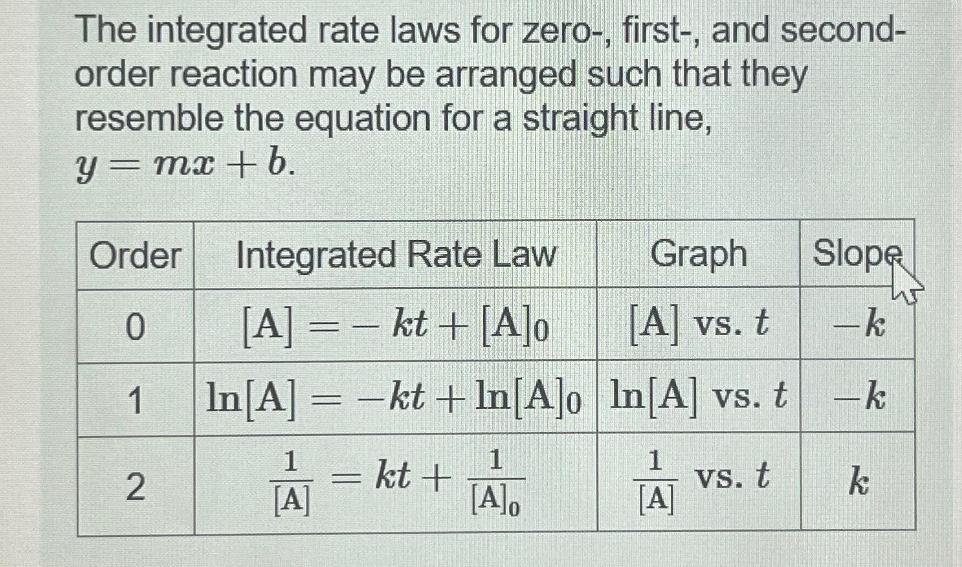
The integrated rate laws for zero-, first-, and second- order reaction may be arranged such that they resemble the equation for a straight line, = mx + b. y = Order 0 1 2 Integrated Rate Law [A] = - kt + [A]o In [A] = -kt+In[A]o 1 [A] - kt+ 1 [A]o Graph [A] vs. t In[A] vs. t 1 vs. t [A] Slope -k -k k
Step by Step Solution
There are 3 Steps involved in it
Step: 1
A A2 kt2t1 A1 150102 k300135 8102 k 394104 Ms B ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



