Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Iodine Clock reaction. Could anyone please help me with the calculations for data table 1 and 2 the values are wrong. The Iodine Clock
The Iodine Clock reaction. 

Could anyone please help me with the calculations for data table 1 and 2 the values are wrong.
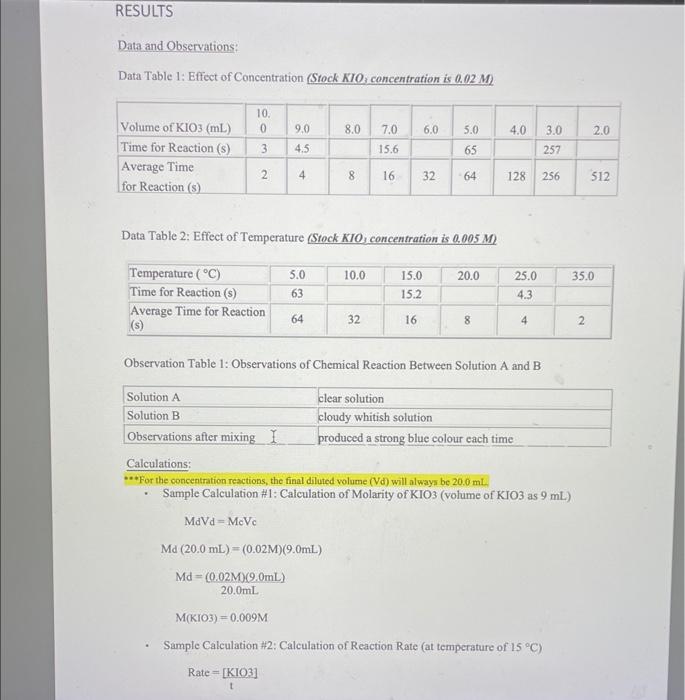
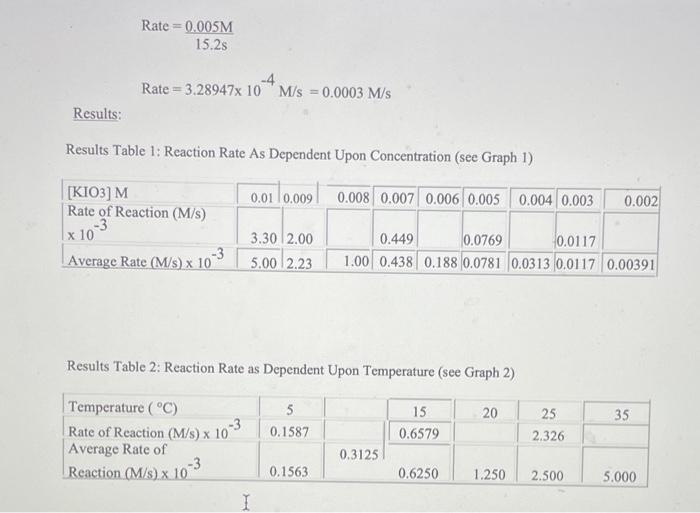
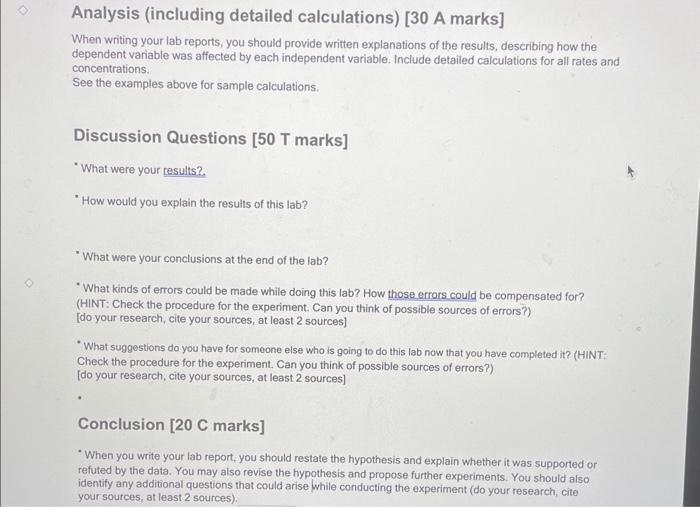
The Iodine Clock Reaction Introduction: The factors that affect the rate of a chemical reaction are important to understand due to the importance of many such reactions to our health, well-being and comfort. It would be advantageous to slow down some of these reactions such as food spoilage and rust formations, while in the cases of reactions such as the Tums-stomach acid reaction and the conversion of organic matter to fossil fuels, it would be beneficial to them speed up. The rate of a reaction is governed by the collision theory. In order for a reaction to occur, there must be a collision between reactant molecules. This collision must have enough energy to break and form the appropriate bonds as well as have the correct orientation when colliding.(1) When all of these things happen, a chemical reaction has occurred. If we can increase the amount of these favourable collisions, then we increase the rate of the reaction.(1) One of the factors that affects reaction rate is reactant concentration. The more reactant molecules in a given area, the greater the number of collisions possible.(1) Whenever we have a larger number of collisions possible, there is a probability of having a favourable collision increases. The opposite is true if we have a lower concentration of reactant molecules. Another factor that affects the rate of reaction is temperature. In this case, there are not more reactant molecules to collide. Temperature is proportional to the average kinetic energy, which is the energy associated with motion.(2) All reactions have what is called the Minimum Threshold Energy. This is the amount of kinetic energy it takes for reactant bonds to break, re-arrange and form the bonds necessary to make products.(1) This energy is equivalent to the activation energy of the reaction. When we increase the temperature of a reaction, the average kinetic energy of the reactants increase.(1) This change results in two things. First, the molecules show an increase in motion within the confines of the same area causing an increase in the amount of collisions. Second, more molecules now have an energy equal to the Minimum Threshold Energy and can now form products. More collisions, more energy, and a greater probability of favourable collisions leads to an increase in reaction rate. Again, the opposite is true if we decrease the temperature. Purpose/Objective: The purpose of this lab is to observe the effect of temperature and concentration on the rate of a chemical reaction. (OR The objective of this lab is to observe the effect of temperature and concentration on the rate of a chemical reaction.) Data and Observations: Data Table 1: Effect of Concentration ( Srock KIO3 concentration is 0.02M ) Data Table 2: Effect of Temperature (Stock KIO i concentration is 0,005M. Observation Table 1: Observations of Chemical Reaction Between Solution A and B Calculations: **For the concentration reactions, the final diluted volume (Vd) will always be 20.0mL. - Sample Calculation \#1: Calculation of Molarity of KIO3 (volume of KIO3 as 9mL ) MdVd=McVcMd(20.0mL)=(0.02M)(9.0mL)Md=(0.02M)(9.0mL)20.0mLM(KIO)=0.009M - Sample Calculation \#2: Calculation of Reaction Rate (at temperature of 15C ) Rate=t[KIO3] Rate=15.2s0.005M Rate=3.28947104M/s=0.0003M/s Results: Results Table 1: Reaction Rate As Dependent Upon Concentration (see Graph 1) Results Table 2: Reaction Rate as Dependent Upon Temperature (see Graph 2) Analysis (including detailed calculations) [ 30 A marks] When writing your lab reports; you should provide written explanations of the results, describing how the dependent variable was affected by each independent variable. Include detailed calculations for all rates and concentrations. See the examples above for sample calculations. Discussion Questions [50 T marks] "What were your results? "How would you explain the results of this lab? -What were your conclusions at the end of the lab? "What kinds of errors could be made while doing this lab? How those errors could be compensated for? (HINT: Check the procedure for the experiment. Can you think of possible sources of errors?) [do your research, cite your sources, at least 2 sources] "What suggestions do you have for someone else who is going to do this lab now that you have completed it? (HINT: Check the procedure for the experiment. Can you think of possible sources of errors?) [do your research, cite your sources, at least 2 sources] Conclusion [20 C marks] - When you write your lab report, you should restate the hypothesis and explain whether it was supported or refuted by the data. You may also revise the hypothesis and propose further experiments. You should also identify any additional questions that could arise while conducting the experiment (do your research, cite your sources, at least 2 sources). The Iodine Clock Reaction Introduction: The factors that affect the rate of a chemical reaction are important to understand due to the importance of many such reactions to our health, well-being and comfort. It would be advantageous to slow down some of these reactions such as food spoilage and rust formations, while in the cases of reactions such as the Tums-stomach acid reaction and the conversion of organic matter to fossil fuels, it would be beneficial to them speed up. The rate of a reaction is governed by the collision theory. In order for a reaction to occur, there must be a collision between reactant molecules. This collision must have enough energy to break and form the appropriate bonds as well as have the correct orientation when colliding.(1) When all of these things happen, a chemical reaction has occurred. If we can increase the amount of these favourable collisions, then we increase the rate of the reaction.(1) One of the factors that affects reaction rate is reactant concentration. The more reactant molecules in a given area, the greater the number of collisions possible.(1) Whenever we have a larger number of collisions possible, there is a probability of having a favourable collision increases. The opposite is true if we have a lower concentration of reactant molecules. Another factor that affects the rate of reaction is temperature. In this case, there are not more reactant molecules to collide. Temperature is proportional to the average kinetic energy, which is the energy associated with motion.(2) All reactions have what is called the Minimum Threshold Energy. This is the amount of kinetic energy it takes for reactant bonds to break, re-arrange and form the bonds necessary to make products.(1) This energy is equivalent to the activation energy of the reaction. When we increase the temperature of a reaction, the average kinetic energy of the reactants increase.(1) This change results in two things. First, the molecules show an increase in motion within the confines of the same area causing an increase in the amount of collisions. Second, more molecules now have an energy equal to the Minimum Threshold Energy and can now form products. More collisions, more energy, and a greater probability of favourable collisions leads to an increase in reaction rate. Again, the opposite is true if we decrease the temperature. Purpose/Objective: The purpose of this lab is to observe the effect of temperature and concentration on the rate of a chemical reaction. (OR The objective of this lab is to observe the effect of temperature and concentration on the rate of a chemical reaction.) Data and Observations: Data Table 1: Effect of Concentration ( Srock KIO3 concentration is 0.02M ) Data Table 2: Effect of Temperature (Stock KIO i concentration is 0,005M. Observation Table 1: Observations of Chemical Reaction Between Solution A and B Calculations: **For the concentration reactions, the final diluted volume (Vd) will always be 20.0mL. - Sample Calculation \#1: Calculation of Molarity of KIO3 (volume of KIO3 as 9mL ) MdVd=McVcMd(20.0mL)=(0.02M)(9.0mL)Md=(0.02M)(9.0mL)20.0mLM(KIO)=0.009M - Sample Calculation \#2: Calculation of Reaction Rate (at temperature of 15C ) Rate=t[KIO3] Rate=15.2s0.005M Rate=3.28947104M/s=0.0003M/s Results: Results Table 1: Reaction Rate As Dependent Upon Concentration (see Graph 1) Results Table 2: Reaction Rate as Dependent Upon Temperature (see Graph 2) Analysis (including detailed calculations) [ 30 A marks] When writing your lab reports; you should provide written explanations of the results, describing how the dependent variable was affected by each independent variable. Include detailed calculations for all rates and concentrations. See the examples above for sample calculations. Discussion Questions [50 T marks] "What were your results? "How would you explain the results of this lab? -What were your conclusions at the end of the lab? "What kinds of errors could be made while doing this lab? How those errors could be compensated for? (HINT: Check the procedure for the experiment. Can you think of possible sources of errors?) [do your research, cite your sources, at least 2 sources] "What suggestions do you have for someone else who is going to do this lab now that you have completed it? (HINT: Check the procedure for the experiment. Can you think of possible sources of errors?) [do your research, cite your sources, at least 2 sources] Conclusion [20 C marks] - When you write your lab report, you should restate the hypothesis and explain whether it was supported or refuted by the data. You may also revise the hypothesis and propose further experiments. You should also identify any additional questions that could arise while conducting the experiment (do your research, cite your sources, at least 2 sources) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


