Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The irreversible, elementary, liquid-phase, exothermic reaction: k1 Additional Information: A occurs in a CSTR with a cooling jacket. The feed is an equimolar mixture

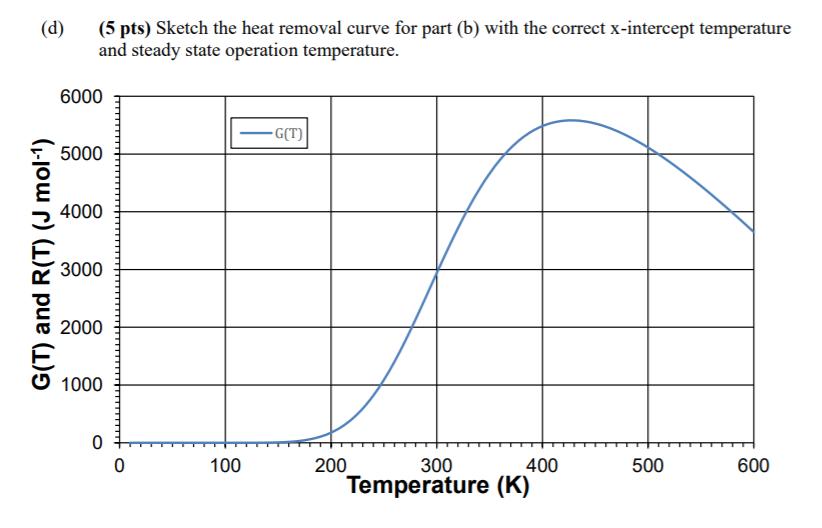

The irreversible, elementary, liquid-phase, exothermic reaction: k1 Additional Information: A occurs in a CSTR with a cooling jacket. The feed is an equimolar mixture of A and I (an inert). FA060 mol min- CAO 10 mol L To = 100 K T = 0.2 s 2B EA,1/R= 2000 K -1 A = 1644 S AHR (298 K) = -10 kJ mol- UA = 25 J (K*s)- Ta = 450 K CpA= CpB= 20 J (mol*K)- f(T) Cpl = 5 J (mol*K)- #f(T) (b) (8 pts) Write the two equations necessary to solve for XA and T using the above information. Do not solve these equations. These equations should be written only in terms of X and T (substitute values and units for all other constants). (Hint: is AC equal to zero? If not, is AHR = AHR at all temperatures?) (d) (5 pts) Sketch the heat removal curve for part (b) with the correct x-intercept temperature and steady state operation temperature. G(T) and R(T) (J mol-) 6000 5000 4000 3000 2000 1000 0 0 100 -G(T) 200 300 Temperature (K) 400 500 600 (e) (5 pts) Explain the chemical reason why the G(T) function in part (d) reaches a maximum and then decreases, as a function of increasing temperature.
Step by Step Solution
★★★★★
3.36 Rating (140 Votes )
There are 3 Steps involved in it
Step: 1
given reaction Criven A Solution FAO 60 kmolmin CAO 10 molL Activation energy let X 2 B fr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


