Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The irreversible first-order (wrt partial pressure of A) gas-phase reaction: A B is carried out isothermally in a fluidized catalytic CSTR containing 50 kg
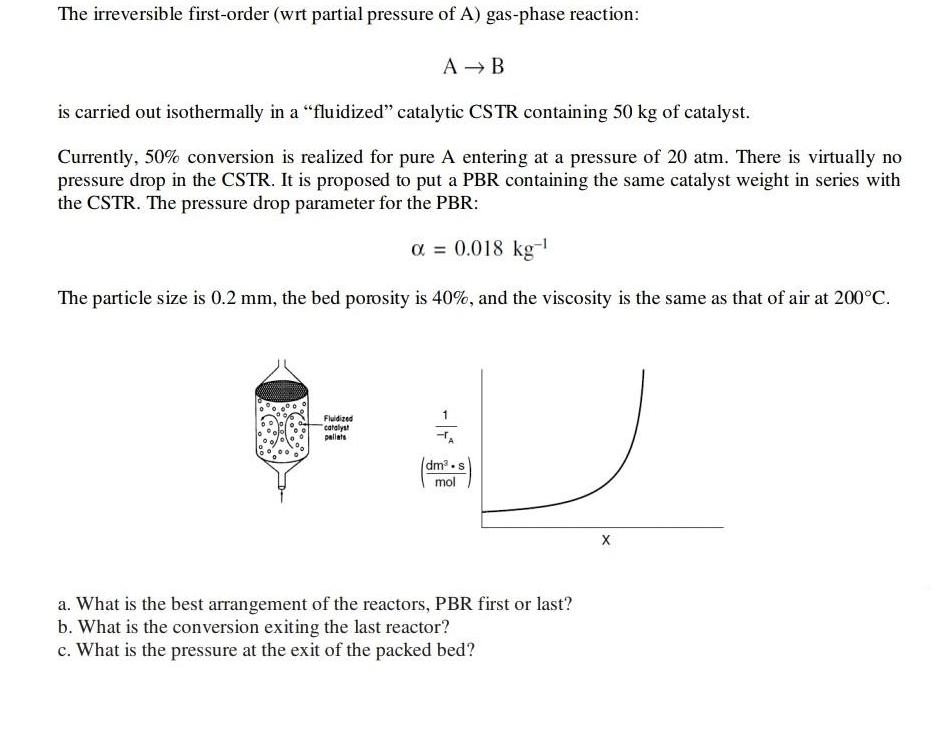
The irreversible first-order (wrt partial pressure of A) gas-phase reaction: A B is carried out isothermally in a "fluidized" catalytic CSTR containing 50 kg of catalyst. Currently, 50% conversion is realized for pure A entering at a pressure of 20 atm. There is virtually no pressure drop in the CSTR. It is proposed to put a PBR containing the same catalyst weight in series with the CSTR. The pressure drop parameter for the PBR: = 0.018 kg- The particle size is 0.2 mm, the bed porosity is 40%, and the viscosity is the same as that of air at 200C. Fluidized catalyst pallats -TA dm-s mol X a. What is the best arrangement of the reactors, PBR first or last? b. What is the conversion exiting the last reactor? c. What is the pressure at the exit of the packed bed?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Step 1 of 4 a The irreversible gasphase reaction is as follows A B The re...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


