Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The isotope of plutonium 238. Pu. Part: 0 / 2 Part 1 of 2 238, Pu is used to make thermoelectric power sources for
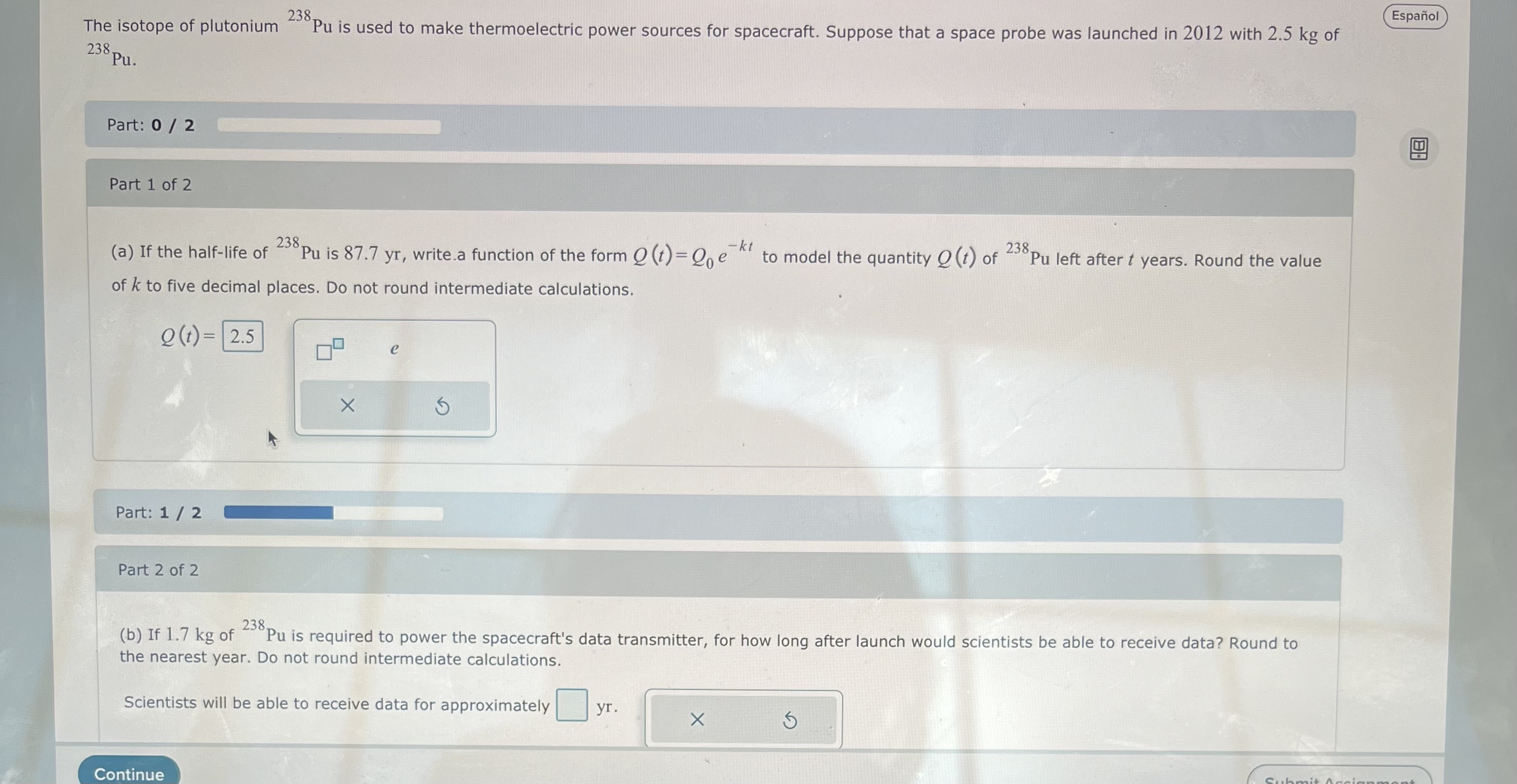
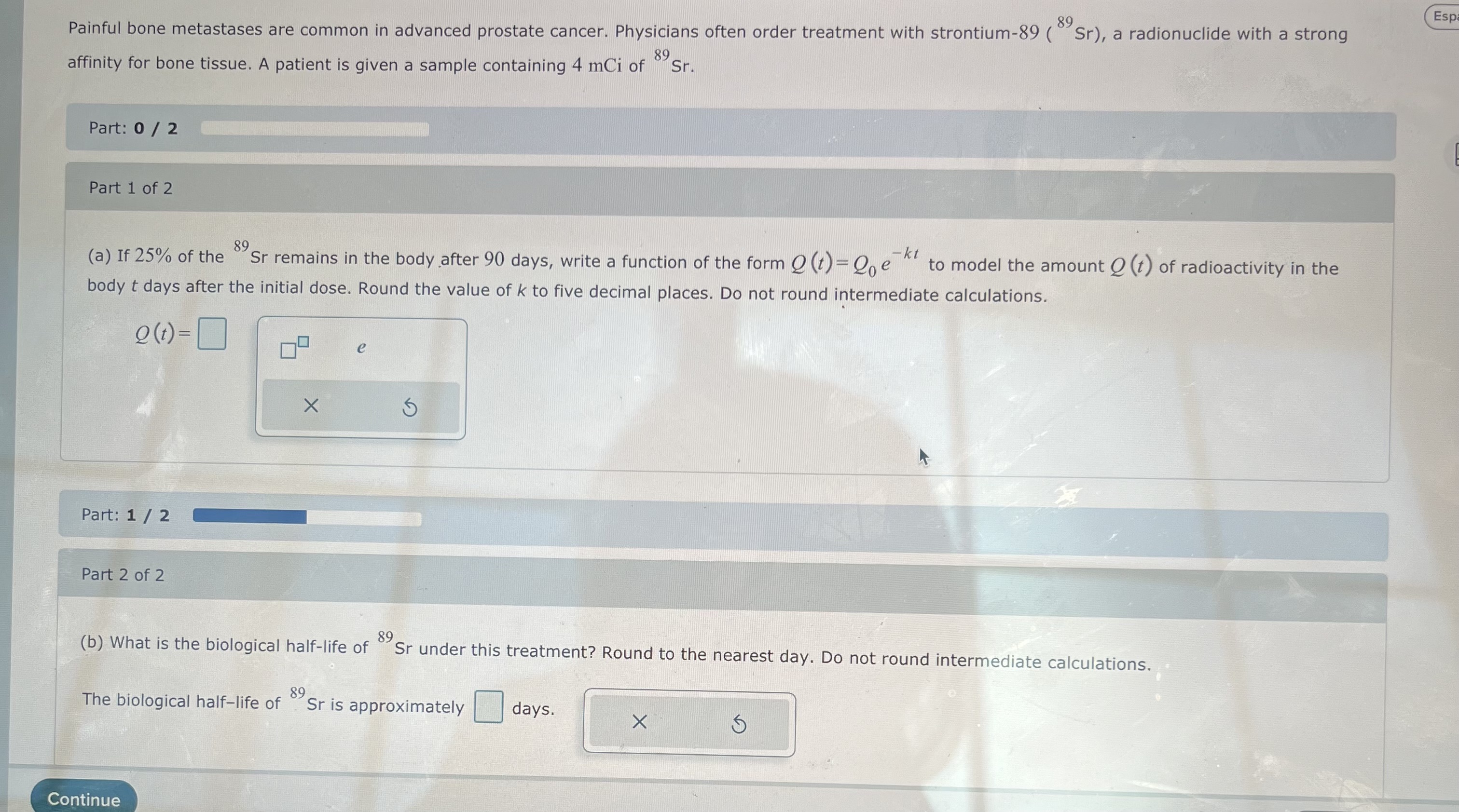
The isotope of plutonium 238. Pu. Part: 0 / 2 Part 1 of 2 238, Pu is used to make thermoelectric power sources for spacecraft. Suppose that a space probe was launched in 2012 with 2.5 kg of 238. (a) If the half-life of Pu is 87.7 yr, write.a function of the form Q(t)=Qe of k to five decimal places. Do not round intermediate calculations. -kt 238. to model the quantity (t) of Pu left after t years. Round the value Q(t)= 2.5 Part: 1/2 Part 2 of 2 e (b) If 1.7 kg of Pu is required to power the spacecraft's data transmitter, for how long after launch would scientists be able to receive data? Round to the nearest year. Do not round intermediate calculations. 238. Scientists will be able to receive data for approximately yr. Continue Espaol Submit Accia B 89 Painful bone metastases are common in advanced prostate cancer. Physicians often order treatment with strontium-89 (Sr), a radionuclide with a strong affinity for bone tissue. A patient is given a sample containing 4 mCi of 89 Sr. Part: 0 / 2 Part 1 of 2 89 (a) If 25% of the Sr remains in the body after 90 days, write a function of the form Q (t)=Qe to model the amount Q (t) of radioactivity in the body t days after the initial dose. Round the value of k to five decimal places. Do not round intermediate calculations. -kt Q(t)= e Part: 1/2 Part 2 of 2 89 (b) What is the biological half-life of Sr under this treatment? Round to the nearest day. Do not round intermediate calculations. 89 The biological half-life of Sr is approximately days. x Continue Espa
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


