Question
The Ka for the H2PO4-/HPO42- buffer is given by the equation: Ka= ([HPO4^-2]gamma [H^+])/[H2PO4^-]=10^-7.20 What is the pH of a 2:3 mixture of H2PO4-
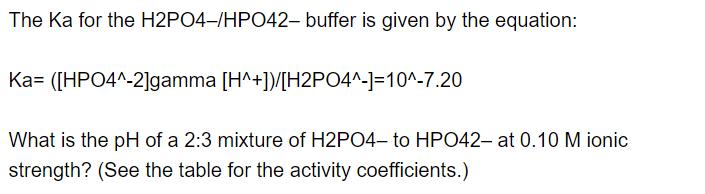
The Ka for the H2PO4-/HPO42- buffer is given by the equation: Ka= ([HPO4^-2]gamma [H^+])/[H2PO4^-]=10^-7.20 What is the pH of a 2:3 mixture of H2PO4- to HPO42- at 0.10 M ionic strength? (See the table for the activity coefficients.)
Step by Step Solution
3.40 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
720 Ka 10 log ka log 631 x 108 pka 720 Now using value for activ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Economics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



