Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The last 2 pages is what's is asking for, though I don't know how to solve for those. everything before that is just instructions for



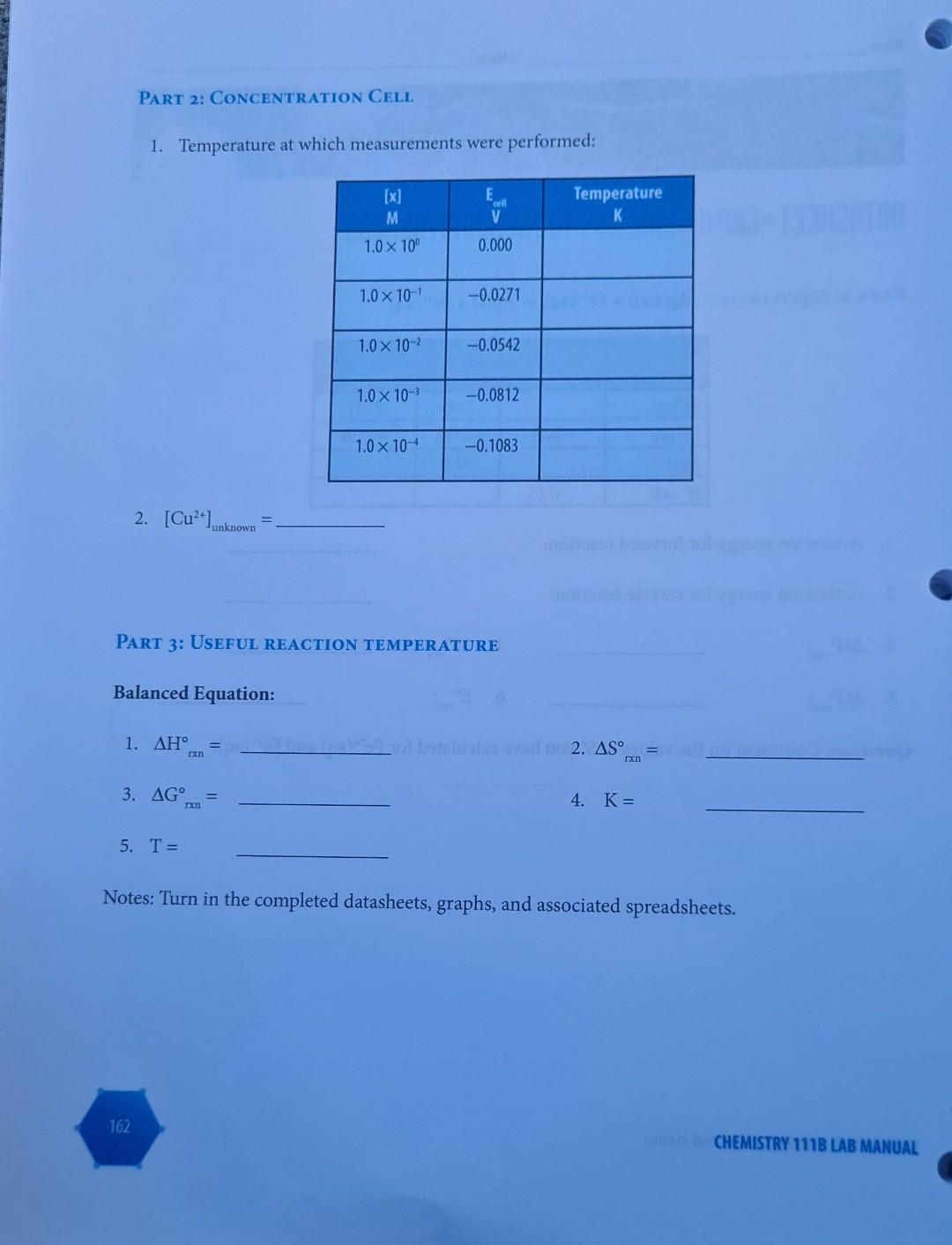
The last 2 pages is what's is asking for, though I don't know how to solve for those. everything before that is just instructions for how to solve it and the information needed to solve it.
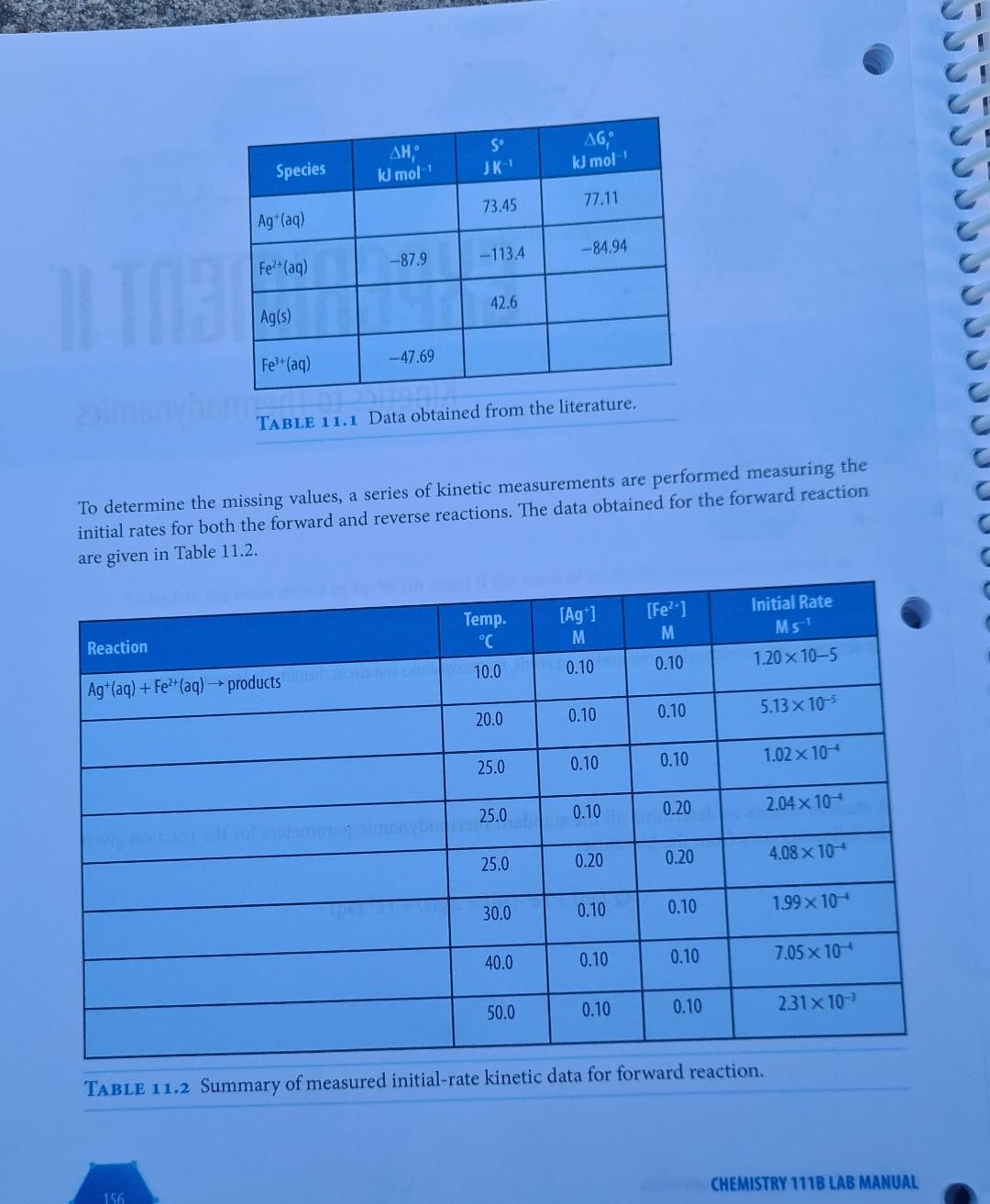
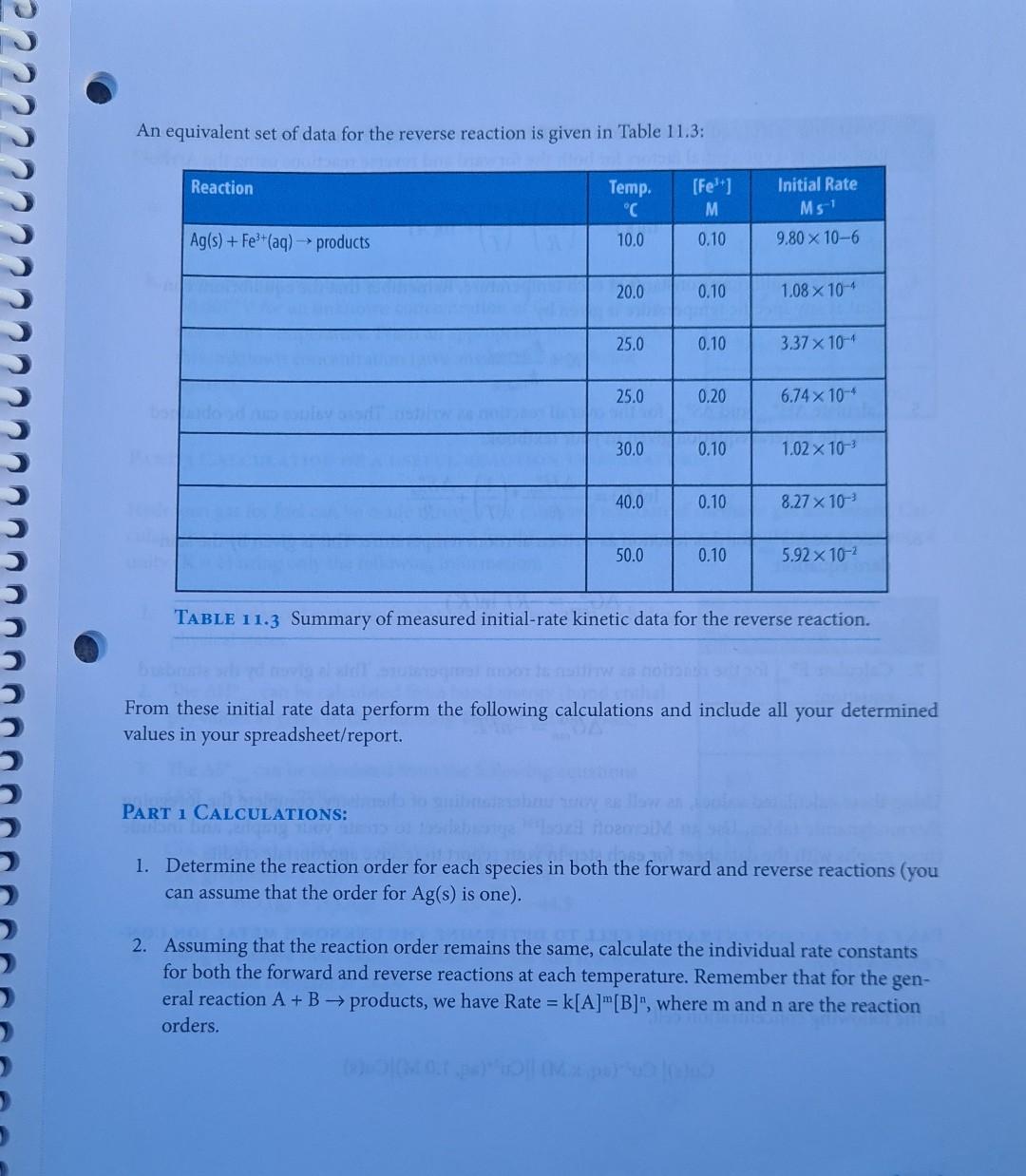
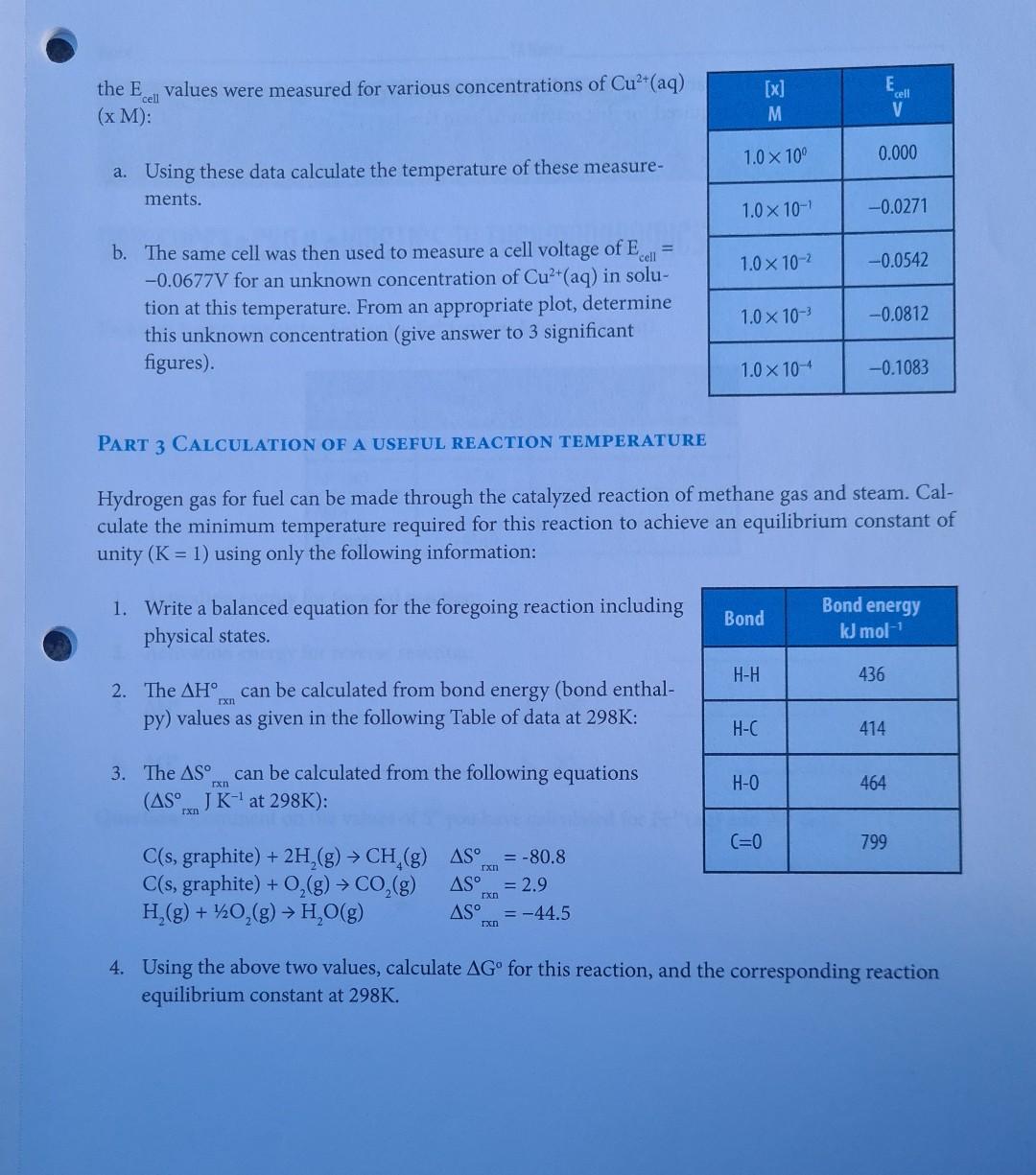
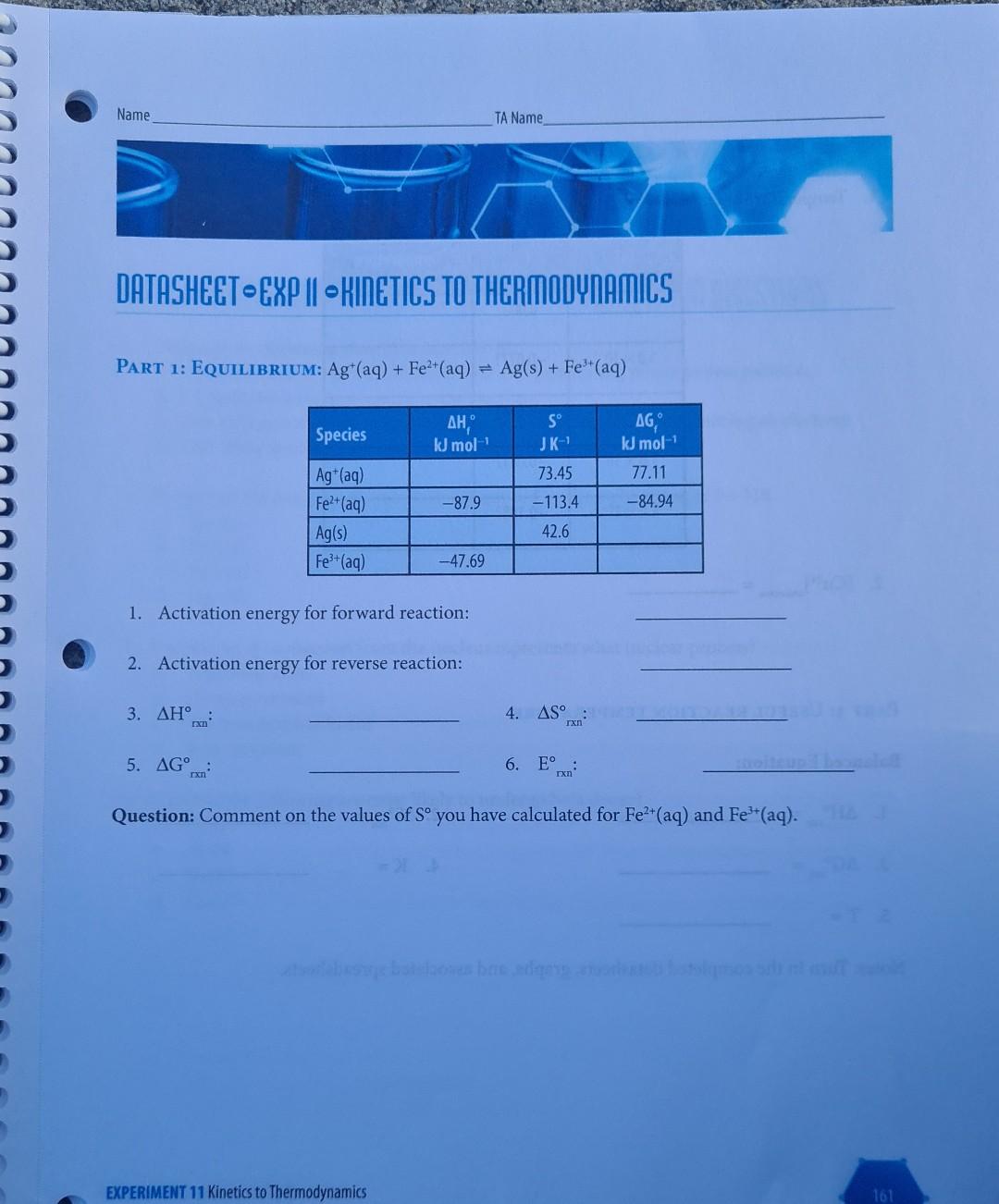
EXPERIMENT I Kinetics to Thermodynamics LEARNING OBJECTIVE To determine the connections between kinetics, equilibria, thermodynamics and electrochemistry in aqueous systems. A student wishes to determine all the standard thermodynamic parameters for the reaction given the following data from the literature. Ag+ (aq) + Fe+ (aq) = Ag(s) + Fe+ (aq) EXPERIMENT 11 Kinetics to Thermodynamics 155 AH, kJ mol- S JK- AG, kJ mol- 73.45 77.11 Ag+ (aq) -87.9 Fe+ (aq) -84.94 -113.4 IL TOBI 42.6 Ag(s) Fe+ (aq) -47.69 m TABLE 11.1 Data obtained from the literature. To determine the missing values, a series of kinetic measurements are performed measuring the initial rates for both the forward and reverse reactions. The data obtained for the forward reaction are given in Table 11.2. [Ag+] [Fe+] Initial Rate Ms Temp. C Reaction M M 10.0 0.10 0.10 1.20 x 10-5 Ag+ (aq) + Fe+ (aq) productsusasio bus zalm 20.0 0.10 0.10 5.13 x 10-5 25.0 0.10 0.10 1.02 x 10- 25.0 0.10 0.20 2.04 x 10 25.0 0.20 0.20 4.08 10 30.0 0.10 0.10 1.99 x 10 40.0 0.10 0.10 7.05 10- 50.0 0.10 0.10 2.31x10- TABLE 11.2 Summary of measured initial-rate kinetic data for forward reaction. 156 CHEMISTRY 111B LAB MANUAL Species C An equivalent set of data for the reverse reaction is given in Table 11.3: Reaction [Fe+] Initial Rate Temp. C M Ms Ag(s) + Fe+ (aq) products 10.0 0.10 9.80 x 10-6 20.0 0.10 1.08 10-4 25.0 0.10 3.37 10- 25.0 0.20 6.74 x 10-4 30.0 0.10 1.02 10- 40.0 0.10 8.27 x 10- 50.0 0.10 5.92 x 10- TABLE 11.3 Summary of measured initial-rate kinetic data for the reverse reaction. bind entha From these initial rate data perform the following calculations and include all your determined values in your spreadsheet/report. PART 1 CALCULATIONS: 1. Determine the reaction order for each species in both the forward and reverse reactions (you can assume that the order for Ag(s) is one). 2. Assuming that the reaction order remains the same, calculate the individual rate constants for both the forward and reverse reactions at each temperature. Remember that for the gen- eral reaction A + B products, we have Rate = k[A]"[B]", where m and n are the reaction orders. 3. Using your calculated rate constants at different temperatures, determine the activation ener- gies and pre-exponential factors for both the forward and reverse reactions using the Arrhe- nius equation: In(k)= (*)-(-) + + In(A) 4. Calculate equilibrium constants at each temperature. Remember that the equilibrium con- stant at any specific temperature is given by k forward K= k reverse rxn rxn 5. Calculate AH and AS for the overall reaction as written. These values can be obtained from the general equation given in your textbook: In(K)=- ** (1), A.S. -AH R R 6. Calculate AG for this reaction as written at room temperature. This is given by the stan- Ixn dard equation: AG=-RT ln(K) CEREALS 7. Calculate E for the reaction as written at room temperature. This is given by the standard equation: rxn ulon bror eroltsiral AG=-nFE Using these calculated values, as well as your understanding of chemistry, complete the foregoing thermodynamic tables. Use an Microsoft ExcelTM spreadsheet to create your graphs, and include these graphs with the datasheet for each step in your report to receive appropriate credit. PART 2 USE A CONCENTRATION CELL TO DETERMINE THE UNKNOWN METAL ION CON- CENTRATION: In the following concentration cell, Cu(s)| Cu+ (aq, x M) ||Cu+ (aq, 1.0 M) Cu(s) FX71 + the Evalues were measured for various concentrations of Cu2+ (aq) (x M): [x] cell M 1.0 10 0.000 a. Using these data calculate the temperature of these measure- ments. 1.0 10- -0.0271 = 1.0 10- -0.0542 b. The same cell was then used to measure a cell voltage of E cell -0.0677V for an unknown concentration of Cu+ (aq) in solu- tion at this temperature. From an appropriate plot, determine this unknown concentration (give answer to 3 significant figures). 1.0 10- -0.0812 1.0 10-4 -0.1083 PART 3 LCULATION OF A USEFUL REACTION TEMPERATURE Hydrogen gas for fuel can be made through the catalyzed reaction of methane gas and steam. Cal- culate the minimum temperature required for this reaction to achieve an equilibrium constant of unity (K = 1) using only the following information: 1. Write a balanced equation for the foregoing reaction including physical states. Bond Bond energy kJ mol- H-H 436 2. The AH can be calculated from bond energy (bond enthal- rxn py) values as given in the following Table of data at 298K: H-C 414 3. The AS can be calculated from the following equations (AS J K- at 298K): rxn H-O 464 C=0 799 C(s, graphite) + 2H(g) CH(g) AS = -80.8 C(s, graphite) + O(g) CO(g) AS = 2.9 rxn H(g) + 1/2O(g) HO(g) AS = -44.5 rxn 4. Using the above two values, calculate AG for this reaction, and the corresponding reaction equilibrium constant at 298K. E 5. Assuming that AH and AS are independent of temperature, calculate the AS values temperature (in K) required for this reaction to have K = 1. Exn rxn 80s ab to oldal gaiwolle? Name TA Name DATASHEET EXP II KINETICS TO THERMODYNAMICS O ( PART 1: EQUILIBRIUM: Agt (aq) + Fe+ (aq) = Ag(s) + Fe+ (aq) S Species AH, kJ mol- JK- kJ mol- Ag+ (aq) 73.45 77.11 Fe+ (aq) -87.9 -113.4 -84.94 Ag(s) 42.6 Fe+ (aq) -47.69 1. Activation energy for forward reaction: 2. Activation energy for reverse reaction: 3. 4. AS 5. AG 6. E coolteupil rxn Question: Comment on the values of S you have calculated for Fe+ (aq) and Fe+ (aq). atslebesu batelowes bas adgarstovu fotolimos sunt f 161 EXPERIMENT 11 Kinetics to Thermodynamics TXn PART 2: CONCENTRATION CELL 1. Temperature at which measurements were performed: [x] E M V 1.0 10 0.000 1.0 10- -0.0271 1.0 10- -0.0542 1.0 10- -0.0812 1.0 10- -0.1083 2. [Cu+] unknown PART 3: USEFUL REACTION TEMPERATURE Balanced Equation: 1. hay not betaluolss svar 02. AS Ixn 3. AG 4. K = 5. T= Notes: Turn in the completed datasheets, graphs, and associated spreadsheets. 162 Ixn IxI cell Temperature K CHEMISTRY 111B LAB MANUALStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


