The Lead-Tin phase diagram: a) Show the eutectic three phase reaction on this phase diagram. b) A Pb-45% Sn alloy is cooled from 300C
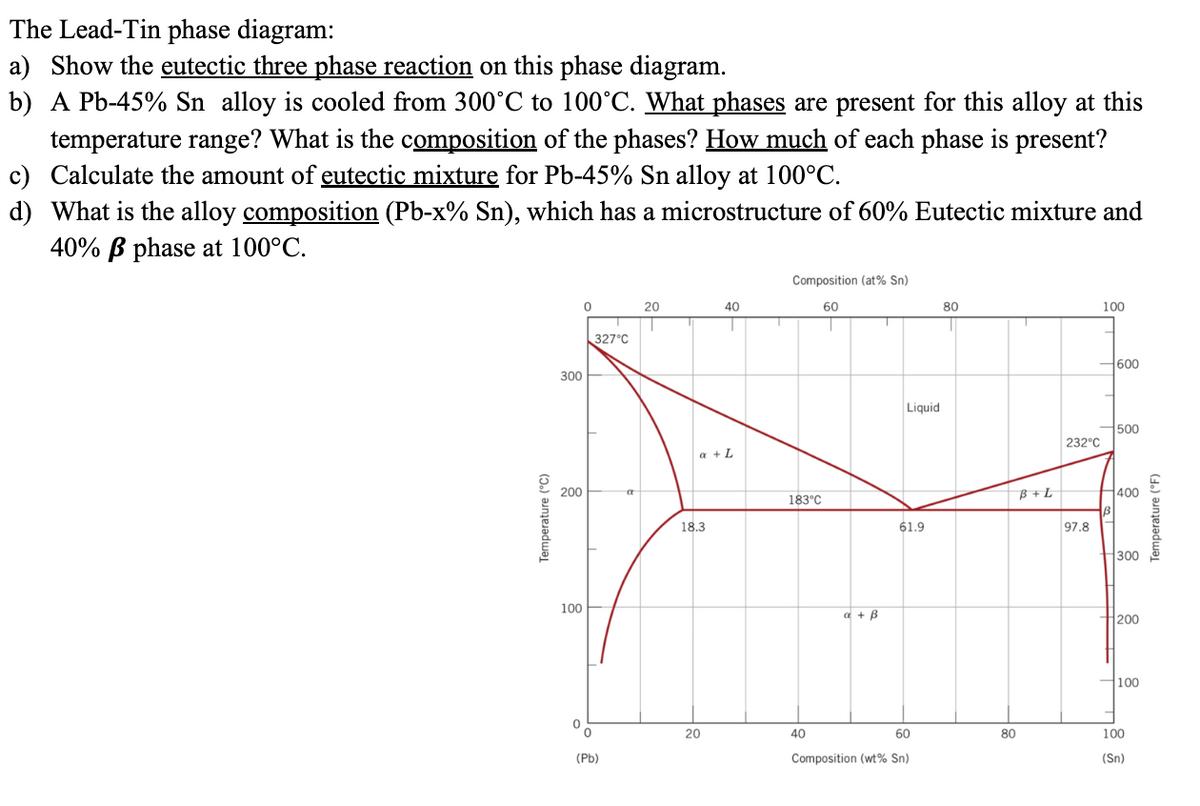
The Lead-Tin phase diagram: a) Show the eutectic three phase reaction on this phase diagram. b) A Pb-45% Sn alloy is cooled from 300C to 100C. What phases are present for this alloy at this temperature range? What is the composition of the phases? How much of each phase is present? c) Calculate the amount of eutectic mixture for Pb-45% Sn alloy at 100C. d) What is the alloy composition (Pb-x% Sn), which has a microstructure of 60% Eutectic mixture and 40% phase at 100C. Composition (at% Sn) 60 0 20 40 80 100 1 Temperature (C) 300 200 100 327C '0 (Pb) a a + L 18.3 20 183C a + B Liquid 61.9 40 60 Composition (wt% Sn) 80 B+L 232C 97.8 600 500 400 300 200 100 100 (Sn) Temperature (F)
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
327c 300C d 1080 xL IG 183C A Liquid 2 B e D BL iX HI 183 1 ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


