Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Lewis representation above depicts a reaction between hydrogen (blue) and a main-group element from group [4A In this representation, each Y atom needs
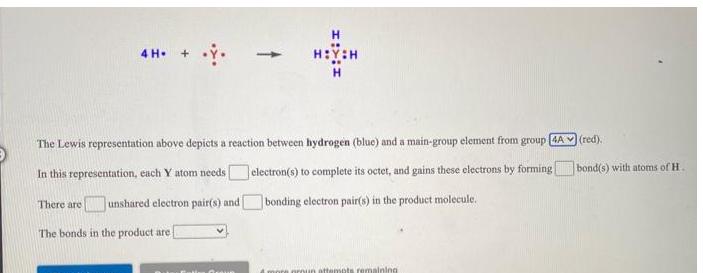
The Lewis representation above depicts a reaction between hydrogen (blue) and a main-group element from group [4A In this representation, each Y atom needs There are unshared electron pair(s) and electron(s) to complete its octet, and gains these electrons by forming bonding electron pair(s) in the product molecule. The bonds in the product are 4 more group attempts remaining (red). bond(s) with atoms of H.
Step by Step Solution
★★★★★
3.50 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


