Question
What volume of a 0.158 M sodium hydroxide solution is required to react with 24.8 ml of a 0.242 M nitric acid solution? Give
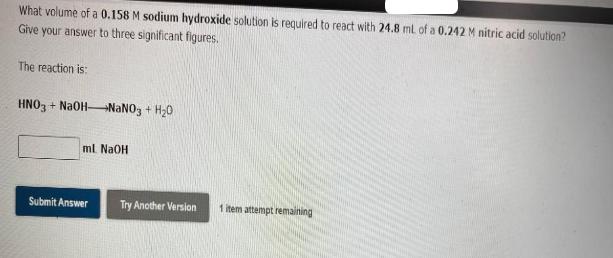
What volume of a 0.158 M sodium hydroxide solution is required to react with 24.8 ml of a 0.242 M nitric acid solution? Give your answer to three significant figures. The reaction is: HNO3 + NaOH-NaNO3 + H0 ml NaOH Submit Answer Try Another Version 1 item attempt remaining
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Given NaOH The concentration The concentration of HNO3 of 248ml Volume of HNO 3 Equivalents ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Advanced Engineering Mathematics
Authors: ERWIN KREYSZIG
9th Edition
0471488852, 978-0471488859
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



