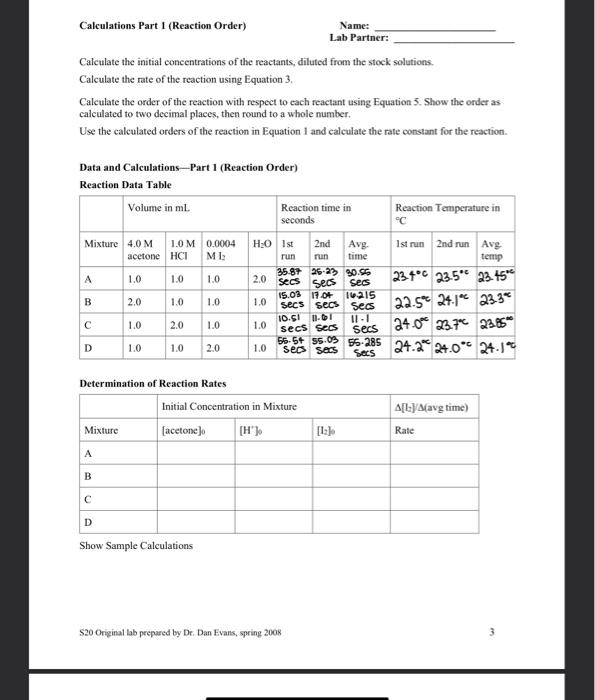
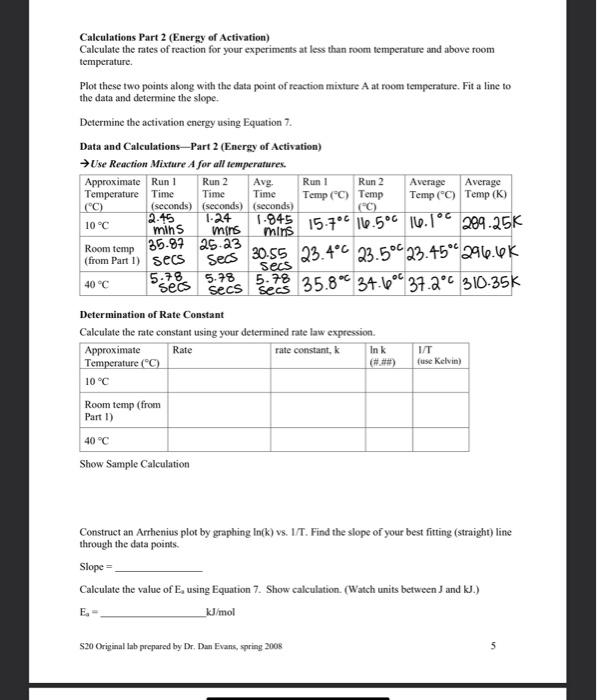
The lodination of Acetone, a Chemical Kinetics Lab Please read the kinetics chapter of the text book for a background on kinetics. Focus on 1) determining orders of reaction and 2) using temperature variation to determine energy of activation. Introduction In this experiment, we will study and determine the kinetics of the reaction between iodine and acetone in aqueous solution: H CH.COCH: + 1 CH.COCH I+H+1 The rate of the reaction is expected to depend on the hydrogen ion concentration, a catalyst, as well as the concentration of the two reactants. The rate law for this reaction is: Rate = k[acetone)"[H"J" 1:1 The reaction can be measured by the visual disappearance of the yellow/brown iodine. (The other reactants are clear.) So the rate is measured as: Rate = -A[1] (2) At t (4) lodine will be the limiting reactant in order to use it as the indicator. Acetone and the hydrogen ion will be kept in excess. The time will be measured for the initial concentration of iodine to completely disappear. The rate under this setting will be: Rate -Alla [1:20 - 0 [12] (3) 1-0 By measuring the rate of reaction under varying concentrations and temperature, we can determine the order of cach species in the rate law and we can determine the activation energy for this reaction. To calculate the order of reaction, we will use the method of initial rates,"even though our rate measurements will be average rates, rather than initial rates. We use two rate measurements where one concentration is doubled and the other concentrations are kept the same. Using doubling actone [A] as the example, we divide the two rate equations: Rate 2 = k[2A]"HT: Rate 2-2A1 2m Rate 1 -k[A"H"]"]" Rate 1 (AT" Taking the logarithm log(rate 2 rate 1) - log (2) - m log2 (5) Determining the energy of activation of a reaction requires measuring rate over a range of temperatures. The natural logarithm of the rate constant, In(k), plotted versus inverse Kelvin temperature, 1/T, should yield a straight line fitting the Arrhenius equation (where InA is the y- intercept: A is the frequency factor.): In(k) --E/RX1/T) + InA (6) The slope of the line is equal to: slope = {EXR) (7) The value of R is 8.314 J/K mol based on the desired units of Joules. s20 Origanal lab prepared by Dr. Dan Evans, spring 2008 The solutions to be used are: Equipment needed will include: 4.0 M acetone small test tubes 1.0 MHCI volumetric pipettes (or burets) 0.0004 MI stopwatch dcionized (DI) water thermometer Procedure Part 1 (Reaction Order) 1, is light sensitive. If in a buret or transfer beaker, you must protect it from light to prevent decomposition. leg. wrap with foil or paper) 1. Select two test tubes that when filled with Dl water have identical color when viewed down the test tube at white paper. One test tube will be the reaction test tube and the other will be the reference test tube. Label the reference and set aside. Empty the reaction test tube and save it for step 3. 2. Based on the volumes listed in the Reaction Rate Data Table, pipet or buret the appropriate volumes of acetone, HCl and Dl water into a clean test tube. Swirl the test tube to mix the reagents. (Autopipette reminders: DO NOT go past the maximum or minimum value clearly indicated on the label of the pipette. Slowly depress the plunger to the first stop and slowly release to get the most accurate amount. If you push all the way down, you are pulling in too much liquid. 3. Using a volumetric pipet or buret, add the lz solution into the reaction test tube. Then, simultaneously start the timer and pour the mixture from step 2 into the reaction test tube. Quickly swirl the test tube to mix or stir with a clean, dry stirring rod. 4. The mixture appears yellow because of the presence of the iodine. The iodine color fades slowly as it reacts. Look down the reaction tube and reference tube and stop the time when the color of the iodine disappears. Measure the temperature of the reaction mixture. Record time and temperature. 5. Measure each reaction mixture twice; the times should differ by no more than 20 seconds for each mixture. You can reuse the same test tubes, rinsing the reaction tube with a small amount of DI water between runs. The temperature must remain relatively constant, within three degrees of the initial run. If these conditions did not occur, repeat the reaction. Procedure Part 2 (Energy of Activation) Using the concentrations for reaction mixture A, repeat the experiment at approximately 10C and 40C. To do this: 1) Prepare a water bath at the desired temperature. 2) Measure the acetone, water, and HCl into a clean test tube. Prepare a second clean test tube with the designated amounts of acetone, water, and HCI. 3) Measure the lz into the reaction test tube. Prepare a second reaction test tube with ls. 4) Place all four of these test tubes in the water bath for ten minutes to allow the temperature in the test tubes to equilibrate to the water temperature. You may have to add ice, cool water, or warm water to keep the desired temperature. 5) Combine one set of solutions as before and time the color change of the reaction. Record the data, including the actual reaction temperature, on the data sheet. Repeat for the second set of solutions. S20 Original lab prepared by Dr. Dan Evans, spring 2008 2 Calculations Part 1 (Reaction Order) Name: Lab Partner: Calculate the initial concentrations of the reactants, diluted from the stock solutions. Calculate the rate of the reaction using Equation 3. Calculate the order of the reaction with respect to cach reactant using Equation 5. Show the order as calculated to two decimal places, then round to a whole number. Use the calculated orders of the reaction in Equation 1 and calculate the rate constant for the reaction Data and Calculations - Part 1 (Reaction Order) Reaction Data Table Volume in ml. Reaction time in Reaction Temperature in seconds "C Mixture 4.0 M 1.0M 0.0004 HO Ist 2nd Avg Ist run 2nd run Avg acetone HCI ME run run time temp A 1.0 35-87 26-2% 30.56 1.0 1.0 234C 23.5" 23.454 secs Sess B 2.0 15.03 1904 1.0 ivais 1.0 secs secs Seos 22.5 24.12 233 1.0 2.0 1.0 10.SI 0.01 1.0 secs Seos secs 24.00 237" 23.85 56-5+ s5.09 D 1.0 1.0 2.0 1.0 55-285 secs secs 24.2 24.0" 24.12 Secs 2.0 sec 1.0 Determination of Reaction Rates Initial Concentration in Mixture Mixture (acetone) A[{:}/A/avg time) Rate [12] A B D Show Sample Calculations $20 Original lab prepared by Dr. Dan Evans, spring 2008 Reaction Order Determination Calculated Reaction Order Order coefficient 2 decimal places m (acetone) () Integer n P () Show Sample Calculation Determination of Rate Constant Using the calculated values of the orders of the reaction along with the rates for each reaction mixture, calculate the rate constant for the reaction using Equation 1. Mixture D Average K Show Sample Calculation B Rate Law Write out the full rate law including all the measured values: Rate S20 Original lab prepared by Dr. Dan Evans, spring 2008 4 of 6 Calculations Part 2 (Energy of Activation) Calculate the rates of reaction for your experiments at less than room temperature and above room temperature, Plot these two points along with the data point of reaction mixture A at room temperature. Fit a line to the data and determine the slope. Determine the activation energy using Equation 7. Data and Calculations-- Part 2 (Energy of Activation) Use Reaction Mixture A for all temperatures. Approximate Run 1 Run 2 Avg. Run 1 Run 2 Average Average Temperature Time Time Time Temp (C) Temp Temp (C) Temp (K) (C) (seconds) (seconds) (seconds) 2.45 1-24 10 C 1-945 15.7C 16.500 10.10 209.25K mins mins mins Room temp 35.87 25-23 (from Part I) secs Secs 30.55 23.4C 23.5C 23.45*296.6K 40C 5.78 seos secs secs 34.6 37.2** 310-35k Determination of Rate Constant Calculate the rate constant using your determined rate law expression Approximate Rate rate constant, Ink IT Temperature (C) (#) fuse Kelvin) 10 C 5.78 5.78 35.8C Room temp (from Part 1) 40C Show Sample Calculation Construct an Arrhenius plot by graphing In(k) vs. 1/1. Find the slope of your best fitting (straight) line through the data points Slope Calculate the value of E, using Equation 7. Show calculation. (Watch units between J and kJ.) _kJ/mol S20 Original lab prepared by Dr. Dan Evans, spring 2008 5 Postlab and Discussion for "lodination of Acetone" Name(s): 1. Discuss the effect, if any, on the rates measured if a student had neglected to protect the la solution from light 2. Using your results from this experiment, calculate the expected rate of reaction for the Trial A concentrations at 50C. 3. We know that I was the limiting reactant in this experiment. We monitored its complete disappearance. What would have been the effect if the other reactants were similar in concentration (perhaps 0.0008 M acetone and 0.0008 M HCI) instead of 4.0 M acetone and 1.0 M HCI? What was the purpose of having a HUGE excess of the excess reactants? 4. A. Tie in to Ch 11 and 12: Draw the chemical structure of acetone. B. Explain why acetone is soluble in water. Complete a "Discussion of this experiment and your results. (Please write on a separate paper.) As always, be sure to identify sources of error in this experiment, analyze your data, and show understanding of concepts investigated in this lab, S20 Original lab prepared by Dr. Dan Evans, spring 2008