Question
Print Question 14 of 14 Hint Calculator The mass spectrum of diisopropyl ether is shown below. relative abundance 100 80 Periodic Table 60 base
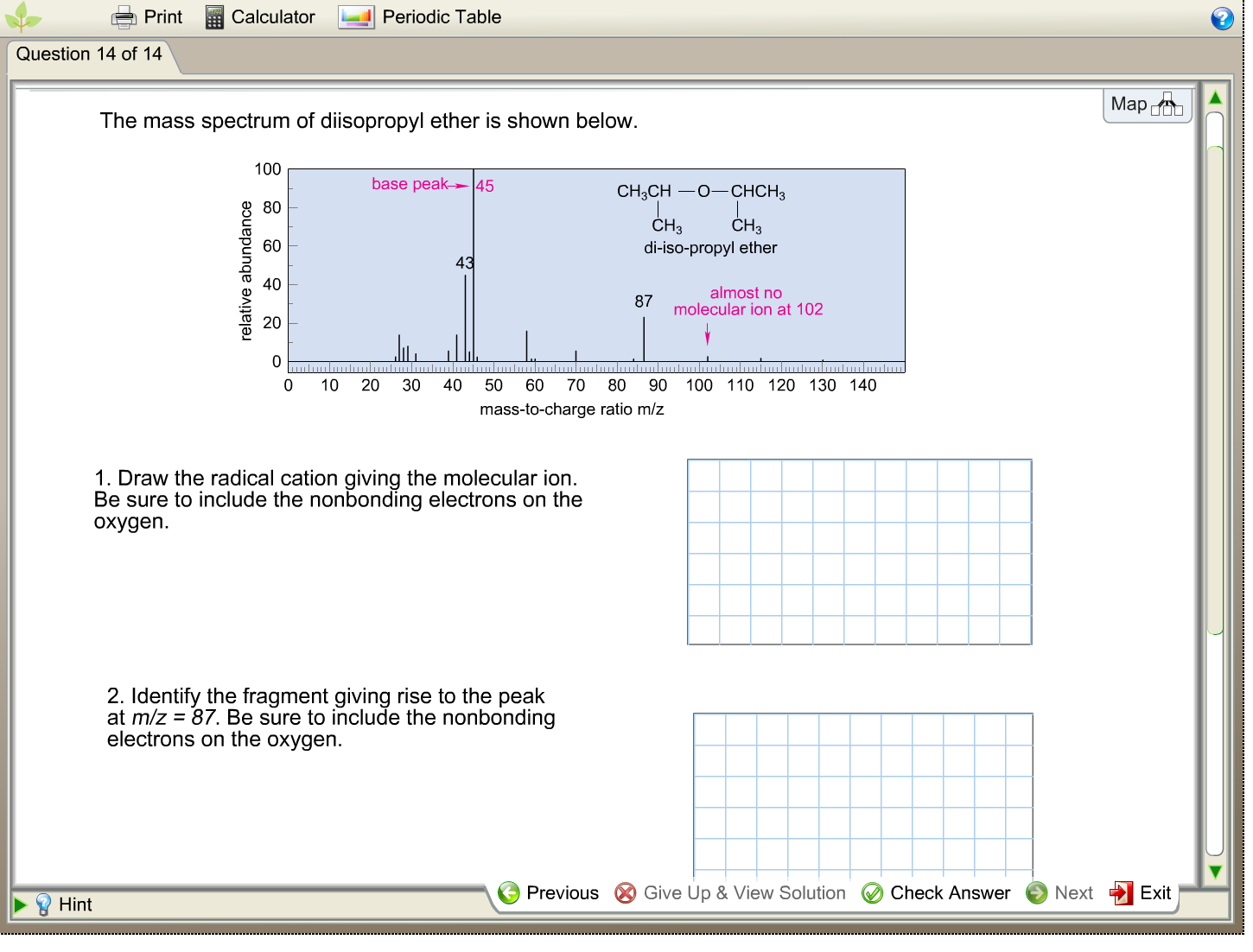
Print Question 14 of 14 Hint Calculator The mass spectrum of diisopropyl ether is shown below. relative abundance 100 80 Periodic Table 60 base peak 45 43 0 10 20 30 40 50 60 70 mass-to-charge 1. Draw the radical cation giving the molecular ion. Be sure to include the nonbonding electrons on the oxygen. 2. Identify the fragment giving rise to the peak at m/z = 87. Be sure to include the nonbonding electrons on the oxygen. Previous CH3CHO-CHCH3 CH3 CH3 di-iso-propyl ether 87 almost no molecular ion at 102 80 90 100 110 120 130 140 ratio m/z Give Up & View Solution Check Answer Next Map DOU Exit
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



