Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The metal niobium melts at a temperature of 2468 C and boils at 4742 C, whereas the metal gallium melts at a temperature of
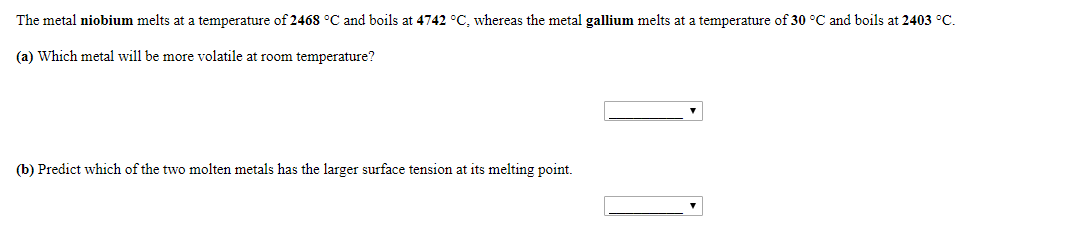
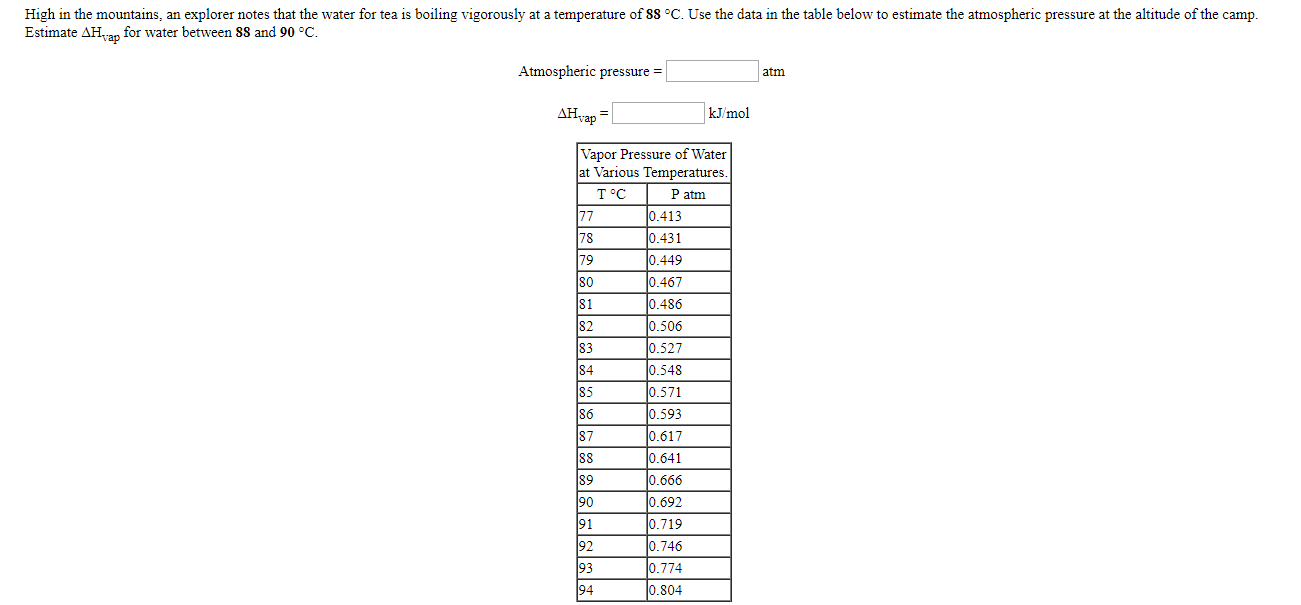
The metal niobium melts at a temperature of 2468 C and boils at 4742 C, whereas the metal gallium melts at a temperature of 30 C and boils at 2403 C. (a) Which metal will be more volatile at room temperature? (b) Predict which of the two molten metals has the larger surface tension at its melting point. High in the mountains, an explorer notes that the water for tea is boiling vigorously at a temperature of 88 C. Use the data in the table below to estimate the atmospheric pressure at the altitude of the camp. Estimate AH vap for water between 88 and 90 C. Atmospheric pressure = atm AH kJ/mol Vapor Pressure of Water at Various Temperatures. TC P atm 77 0.413 78 0.431 79 0.449 80 0.467 81 0.486 82 0.506 83 0.527 84 0.548 85 0.571 86 0.593 87 0.617 88 0.641 89 0.666 90 0.692 91 0.719 92 0.746 93 0.774 94 0.804
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Volatilit y Volatility is a measure of a substances tendency to change into a gas A substance with a high vapor pressure at a given temperature is c...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


