Question
The molecular structure of ethane is shown below; Considering the 3D shape of the molecule and the measured electronegativities of the atoms in this molecule,
The molecular structure of ethane is shown below;
-
Considering the 3D shape of the molecule and the measured electronegativities of the atoms in this molecule, is this a polaron-polar/charged molecule? Explain your answer briefly.
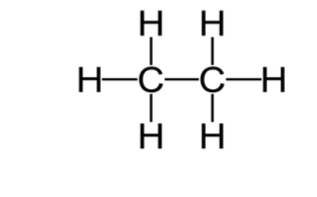
-
The energy required to break the C-C bond in ethane is 450 kJ/mol and the bond length is 1.5 . Assume that we can model the vibrations in this bond by the following harmonic oscillator equation;
// $%&' = $%&'( -) where $%&' = 900 / .
A certain amount of ethane is kept in a container at 500 K. Is the C-C bond likely to break at this temperature? Justify your answer by calculations.
-
Provide a likely percent distortion in the C-C bond one would expect to observe at this temperature. Justify your answer by calculations.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


