Question
Hikers climbing Mount Everest discovered it took much longer to cook a boiled egg than it does at sea level, because the boiling water
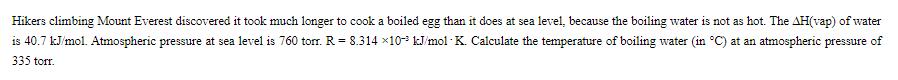
Hikers climbing Mount Everest discovered it took much longer to cook a boiled egg than it does at sea level, because the boiling water is not as hot. The AH(vap) of water is 40.7 kJ/mol. Atmospheric pressure at sea level is 760 torr. R = 8.314 x10 kJ/mol K. Calculate the temperature of boiling water (in C) at an atmospheric pressure of 335 torr.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
International Marketing And Export Management
Authors: Gerald Albaum , Alexander Josiassen , Edwin Duerr
8th Edition
1292016922, 978-1292016924
Students also viewed these General Management questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



