Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The neutral complex [Co(PPh 3 )(NCS) 2 ] was recently published by the group of Cyril Rajnk et. al. along with its magnetic properties. B=0.11
The neutral complex [Co(PPh3)(NCS)2] was recently published by the group of Cyril Rajnk et. al. along with its magnetic properties.
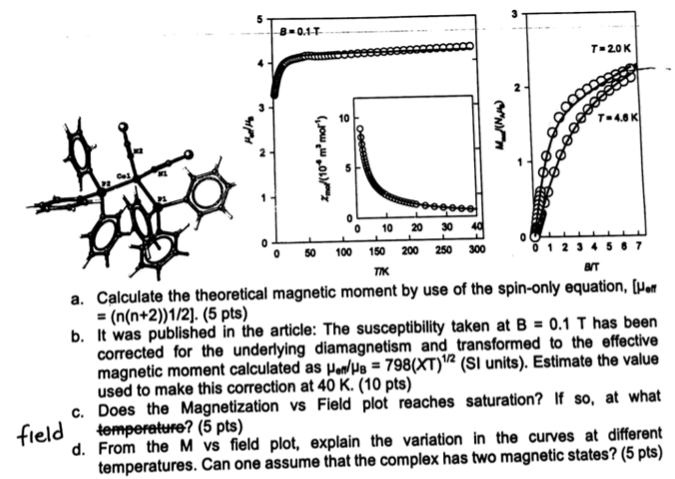
B=0.11 T-20K 40 100 150 300 3 0 10 20 30 50 200 250 TIK BT a. Calculate the theoretical magnetic moment by use of the spin-only equation, (Hot = (n(n+2))1/2). (5 pts) b. It was published in the article: The susceptibility taken at B = 0.1 T has been corrected for the underlying diamagnetism and transformed to the effective magnetic moment calculated as Hen/ We = 798(XT)"? (SI units). Estimate the value used to make this correction at 40 K. (10 pts) C. Does the Magnetization vs Field plot reaches saturation? If so, at what temperature? (5 pts) d. From the M vs field plot, explain the variation in the curves at different temperatures. Can one assume that the complex has two magnetic states? (5 pts) field B=0.11 T-20K 40 100 150 300 3 0 10 20 30 50 200 250 TIK BT a. Calculate the theoretical magnetic moment by use of the spin-only equation, (Hot = (n(n+2))1/2). (5 pts) b. It was published in the article: The susceptibility taken at B = 0.1 T has been corrected for the underlying diamagnetism and transformed to the effective magnetic moment calculated as Hen/ We = 798(XT)"? (SI units). Estimate the value used to make this correction at 40 K. (10 pts) C. Does the Magnetization vs Field plot reaches saturation? If so, at what temperature? (5 pts) d. From the M vs field plot, explain the variation in the curves at different temperatures. Can one assume that the complex has two magnetic states? (5 pts) field
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


