Answered step by step
Verified Expert Solution
Question
1 Approved Answer
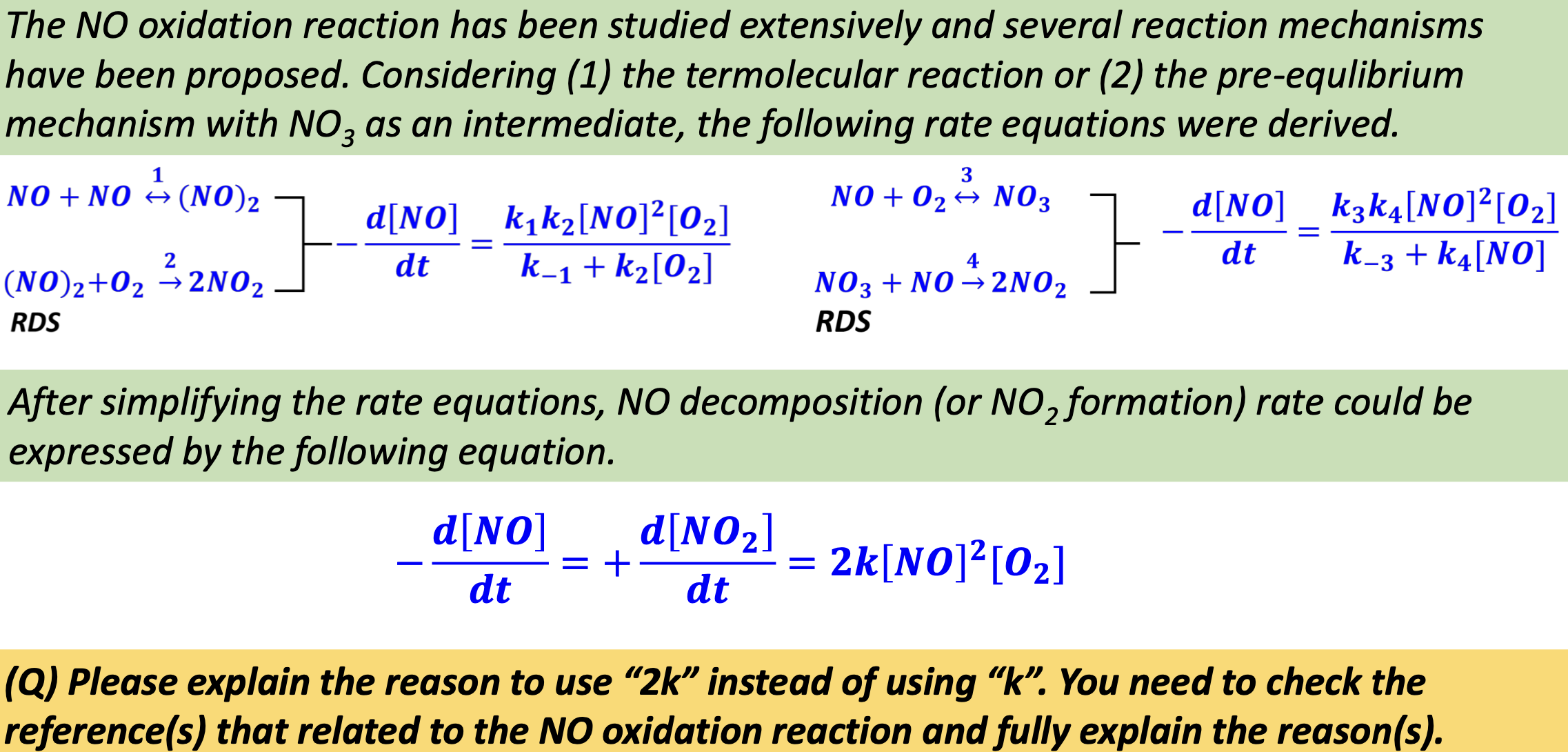
The NO oxidation reaction has been studied extensively and several reaction mechanisms have been proposed. Considering ( 1 ) the termolecular reaction or ( 2
The NO oxidation reaction has been studied extensively and several reaction mechanisms have been proposed. Considering the termolecular reaction or the preequlibrium mechanism with as an intermediate, the following rate equations were derived.
After simplifying the rate equations, decomposition or formation rate could be expressed by the following equation.
Q Please explain the reason to use instead of using You need to check the references that related to the NO oxidation reaction and fully explain the reasons
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


