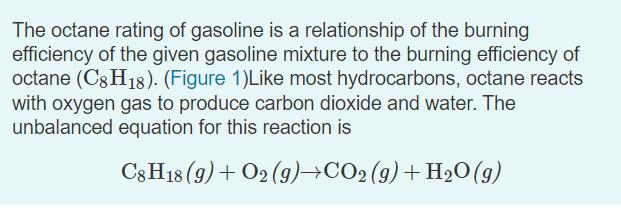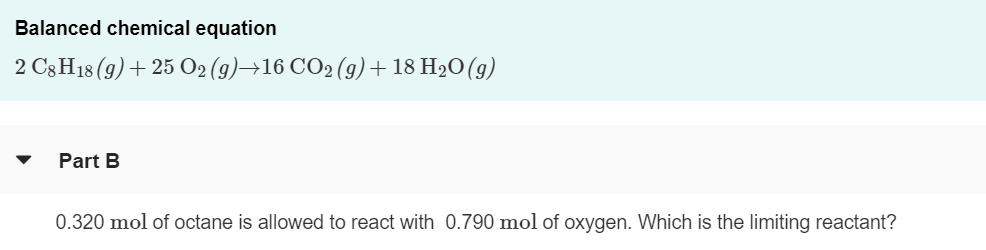Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The octane rating of gasoline is a relationship of the burning efficiency of the given gasoline mixture to the burning efficiency of octane (C8
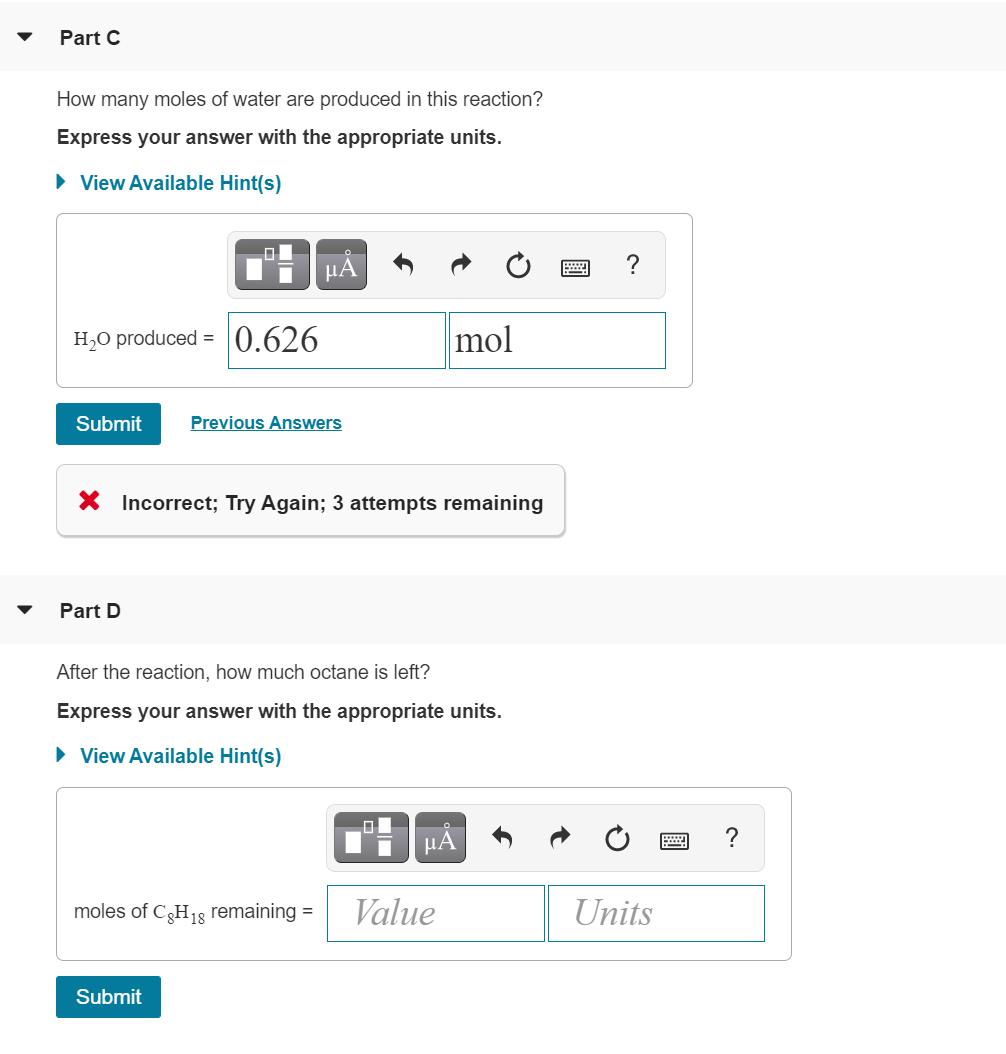
The octane rating of gasoline is a relationship of the burning efficiency of the given gasoline mixture to the burning efficiency of octane (C8 H18). (Figure 1)Like most hydrocarbons, octane reacts with oxygen gas to produce carbon dioxide and water. The unbalanced equation for this reaction is C3 H18 (9) + O2 (9)+CO2 (9)+ H2O(g) Balanced chemical equation 2 C3H18 (9)+ 25 O2 (9)16 CO2 (9) + 18 H2O(g) Part B 0.320 mol of octane is allowed to react with 0.790 mol of oxygen. Which is the limiting reactant? Part C How many moles of water are produced in this reaction? Express your answer with the appropriate units. View Available Hint(s) H ? H,O produced = 0.626 mol Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Part D After the reaction, how much octane is left? Express your answer with the appropriate units. > View Available Hint(s) A moles of CH18 remaining = Value Units Submit The octane rating of gasoline is a relationship of the burning efficiency of the given gasoline mixture to the burning efficiency of octane (C8 H18). (Figure 1)Like most hydrocarbons, octane reacts with oxygen gas to produce carbon dioxide and water. The unbalanced equation for this reaction is C3 H18 (9) + O2 (9)+CO2 (9)+ H2O(g) Balanced chemical equation 2 C3H18 (9)+ 25 O2 (9)16 CO2 (9) + 18 H2O(g) Part B 0.320 mol of octane is allowed to react with 0.790 mol of oxygen. Which is the limiting reactant? Part C How many moles of water are produced in this reaction? Express your answer with the appropriate units. View Available Hint(s) H ? H,O produced = 0.626 mol Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Part D After the reaction, how much octane is left? Express your answer with the appropriate units. > View Available Hint(s) A moles of CH18 remaining = Value Units Submit
Step by Step Solution
★★★★★
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started