Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The only measuring glass in your lab happens to be a shot glass. You pour 8 shots of chilled chloroform (29.18 oF) into your flask
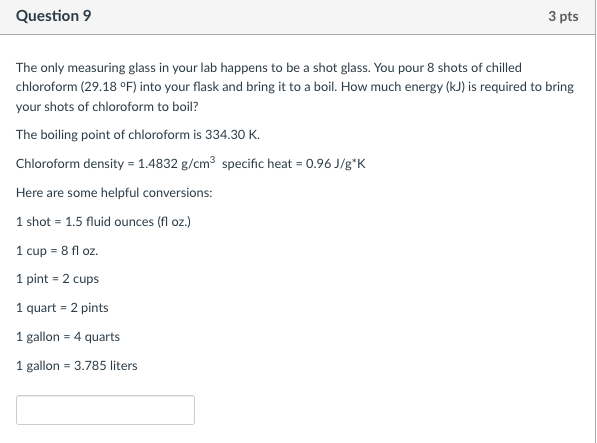
The only measuring glass in your lab happens to be a shot glass. You pour 8 shots of chilled chloroform (29.18 oF) into your flask and bring it to a boil. How much energy (kJ) is required to bring your shots of chloroform to boil? The boiling point of chloroform is 334.30 K. Chloroform density = 1.4832 g/cm3 specific heat = 0.96 J/g*K
Here are some helpful conversions:
1 shot = 1.5 fluid ounces (fl oz.)
1 cup = 8 fl oz.
1 pint = 2 cups
1 quart = 2 pints
1 gallon = 4 quarts
1 gallon = 3.785 liters
Question 9 3 pts The only measuring glass in your lab happens to be a shot glass. You pour 8 shots of chilled chloroform (29.18 F) into your flask and bring it to a boil. How much energy (kJ) is required to bring your shots of chloroform to boil? The boiling point of chloroform is 334.30 K. Chloroform density = 1.4832 g/cm3 specific heat = 0.96J/g*K Here are some helpful conversions: 1 shot = 1.5 fluid Ounces (fl oz.) 1 cup = 8 fl oz. 1 pint = 2 cups 1 quart = 2 pints 1 gallon = 4 quarts 1 gallon - 3.785 litersStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


