Answered step by step
Verified Expert Solution
Question
1 Approved Answer
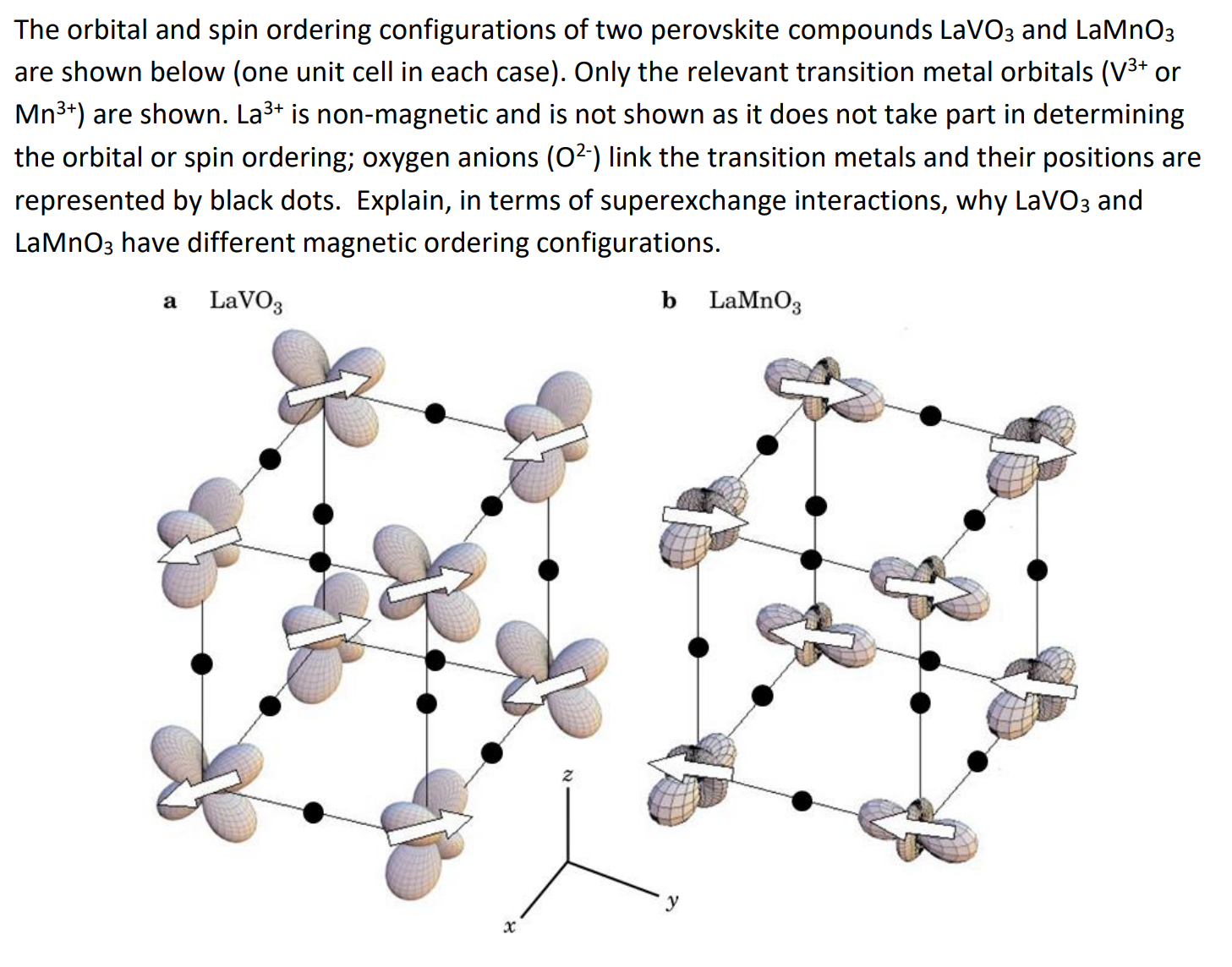
The orbital and spin ordering configurations of two perovskite compounds L a V O 3 and L a M n O 3 are shown below
The orbital and spin ordering configurations of two perovskite compounds and
are shown below one unit cell in each case Only the relevant transition metal orbitals or
are shown. is nonmagnetic and is not shown as it does not take part in determining
the orbital or spin ordering; oxygen anions link the transition metals and their positions are
represented by black dots. Explain, in terms of superexchange interactions, why and
have different magnetic ordering configurations.
a
b
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


