Question
The oxidation of pyrite can be represented by the reaction 4FES, pyrite + 150, + 14H20 4Fe(OH)3 ferrihydraite + 8SO3- + 16H* (a) Write
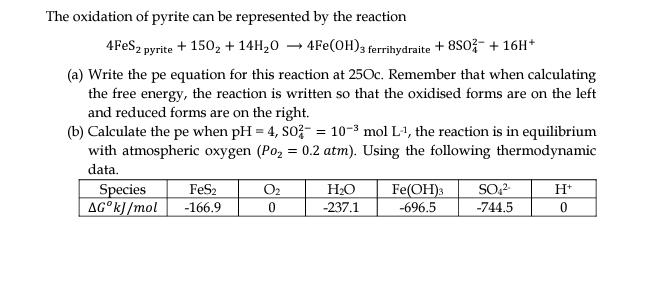
The oxidation of pyrite can be represented by the reaction 4FES, pyrite + 150, + 14H20 4Fe(OH)3 ferrihydraite + 8SO3- + 16H* (a) Write the pe equation for this reaction at 250c. Remember that when calculating the free energy, the reaction is written so that the oxidised forms are on the left and reduced forms are on the right. (b) Calculate the pe when pH = 4, SO? = 10-3 mol L-1, the reaction is in equilibrium with atmospheric oxygen (Po, = 0.2 atm). Using the following thermodynamic %3D data. O2 HO SO,? Species AGK]/mol FeS2 -166.9 Fe(OH)3 -696.5 -237.1 -744.5
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



