Answered step by step
Verified Expert Solution
Question
1 Approved Answer
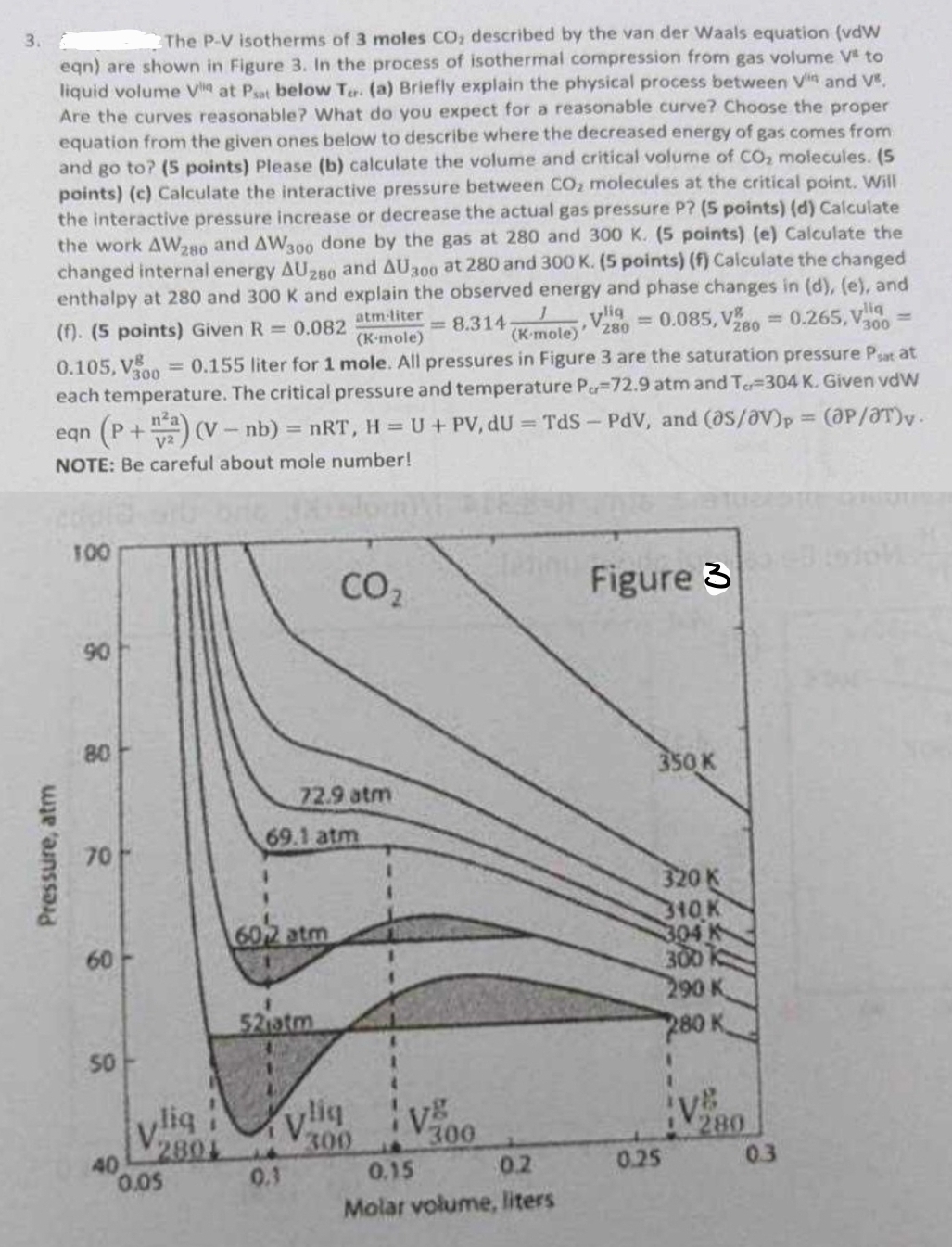
The P - V isotherms of 3 moles C O 2 described by the van der Waals equation ( vdW eqn ) are shown in
The PV isotherms of moles described by the van der Waals equation vdW eqn are shown in Figure In the process of isothermal compression from gas volume to liquid volume at below a Briefly explain the physical process between and Are the curves reasonable? What do you expect for a reasonable curve? Choose the proper equation from the given ones below to describe where the decreased energy of gas comes from and go to points Please b calculate the volume and critical volume of molecules. pointsc Calculate the interactive pressure between molecules at the critical point. Will the interactive pressure increase or decrease the actual gas pressure P pointsd Calculate the work and done by the gas at and pointse Calculate the changed internal energy and at and pointsf Calculate the changed enthalpy at and and explain the observed energy and phase changes in de and f points Given liter for mole. All pressures in Figure are the saturation pressure at each temperature. The critical pressure and temperature atm and Given eqn and elVelT NOTE: Be careful about mole number!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


