Answered step by step
Verified Expert Solution
Question
1 Approved Answer
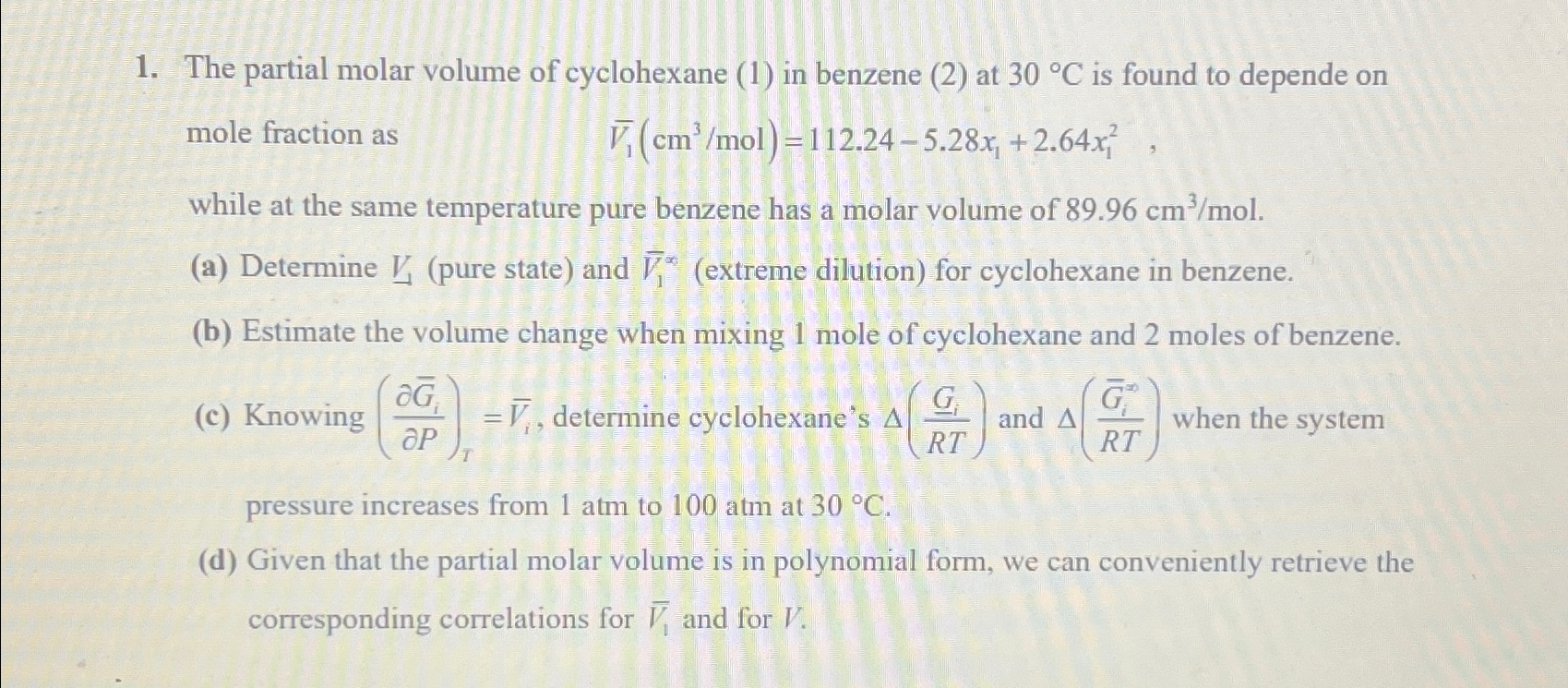
The partial molar volume of cyclohexane ( 1 ) in benzene ( 2 ) at 3 0 C is found to depende on mole fraction
The partial molar volume of cyclohexane in benzene at is found to depende on mole fraction as
while at the same temperature pure benzene has a molar volume of
a Determine pure state and extreme dilution for cyclohexane in benzene.
b Estimate the volume change when mixing mole of cyclohexane and moles of benzene.
c Knowing determine cyclohexane's and when the system pressure increases from atm to atm at
d Given that the partial molar volume is in polynomial form, we can conveniently retrieve the corresponding correlations for and for
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


