Question
The phase diagram of carbon dioxide is below. It is a pretty typical phase diagram showing the temperature and pressure conditions under which CO2
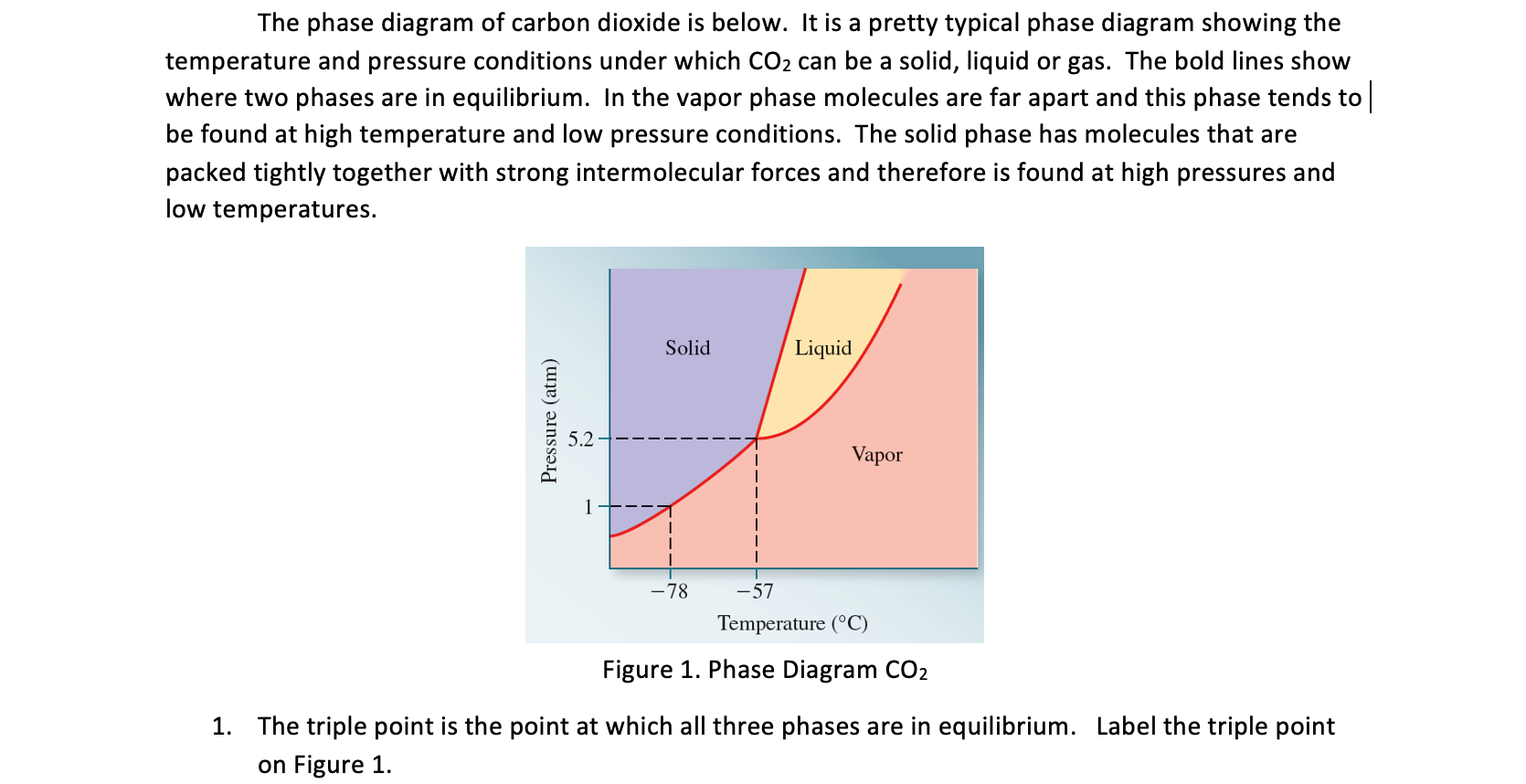
The phase diagram of carbon dioxide is below. It is a pretty typical phase diagram showing the temperature and pressure conditions under which CO2 can be a solid, liquid or gas. The bold lines show where two phases are in equilibrium. In the vapor phase molecules are far apart and this phase tends to be found at high temperature and low pressure conditions. The solid phase has molecules that are packed tightly together with strong intermolecular forces and therefore is found at high pressures and low temperatures. Pressure (atm) 5.2 Solid Liquid -78 -57 Temperature (C) Vapor Figure 1. Phase Diagram CO2 1. The triple point is the point at which all three phases are in equilibrium. Label the triple point on Figure 1.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The phase diagram is a graphical representation of temp...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



