Question
The pk, of hypochloron acid in 7.530. A S40 ml. olution of 012 Msodim ypochorie (NOCied wi 0 M HCL Calculate the pH of
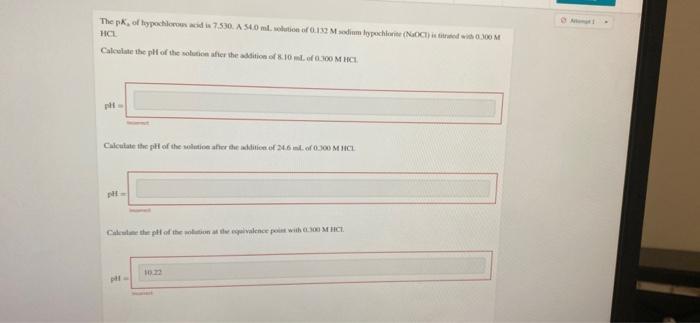
The pk, of hypochloron acid in 7.530. A S40 ml. olution of 012 Msodim ypochorie (NOCied wi 0 M HCL Calculate the pH of the solution afier the addition of S.10mlof000 M HCI Calculate the pt of the solution afher the adkdition of 246 iml. of 0.300 M HCL Caleulae the pi of the solton a te ivalence poit with 00 M HCI. 10.22
Step by Step Solution
3.46 Rating (169 Votes )
There are 3 Steps involved in it
Step: 1
Hypochlorous acid HClO A solution of HClO NaOCl constitutes a buffer and the pH can be determined f...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of biochemistry Life at the Molecular Level
Authors: Donald Voet, Judith G. Voet, Charlotte W. Pratt
4th edition
470547847, 978-0470547847
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



