Question
When octane (C8H18, density = 0.699 g/mL) undergoes complete combustion, it provides energy according to the reaction: 2 C8H18 (1) + 25 O2(g) 16
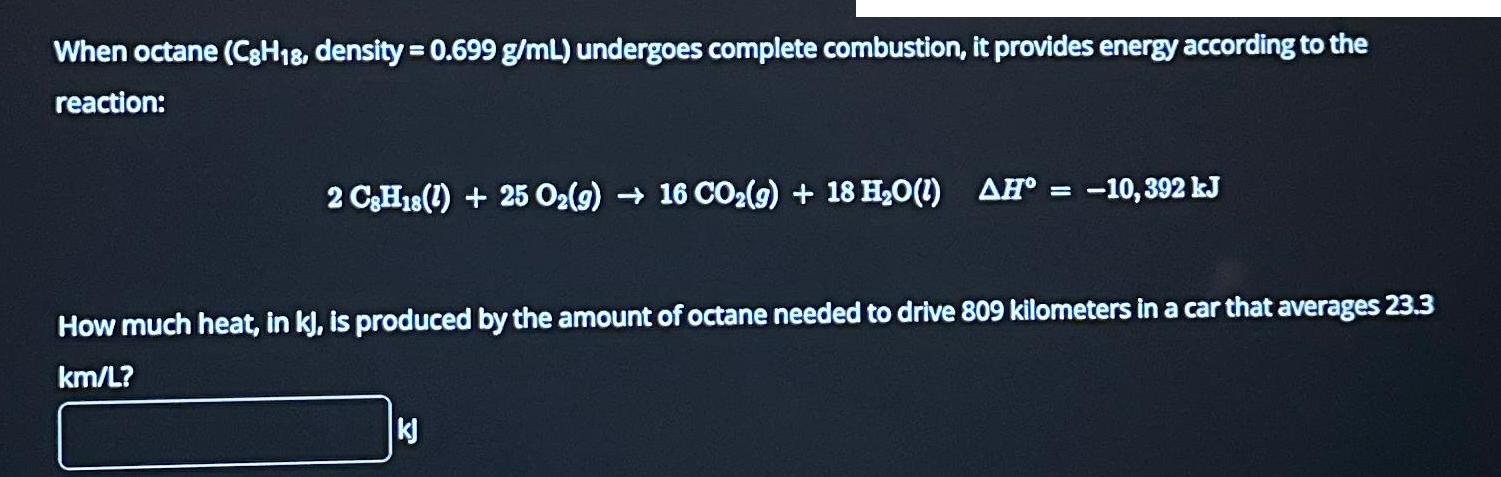
When octane (C8H18, density = 0.699 g/mL) undergoes complete combustion, it provides energy according to the reaction: 2 C8H18 (1) + 25 O2(g) 16 CO(g) + 18 HO(1) = AH -10,392 kJ How much heat, in kj, is produced by the amount of octane needed to drive 809 kilometers in a car that averages 23.3 km/L? kj
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
To solve this problem we need to first calculate the amount of octane needed to drive 809 kilometers ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Economics
Authors: R. Glenn Hubbard
6th edition
978-0134797731, 134797736, 978-0134106243
Students also viewed these Economics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



