Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. In an ammonia converter the fresh feed is 75.16% H, 24.57% N and 0.27% Ar. The fresh feed is mixed with the recycle
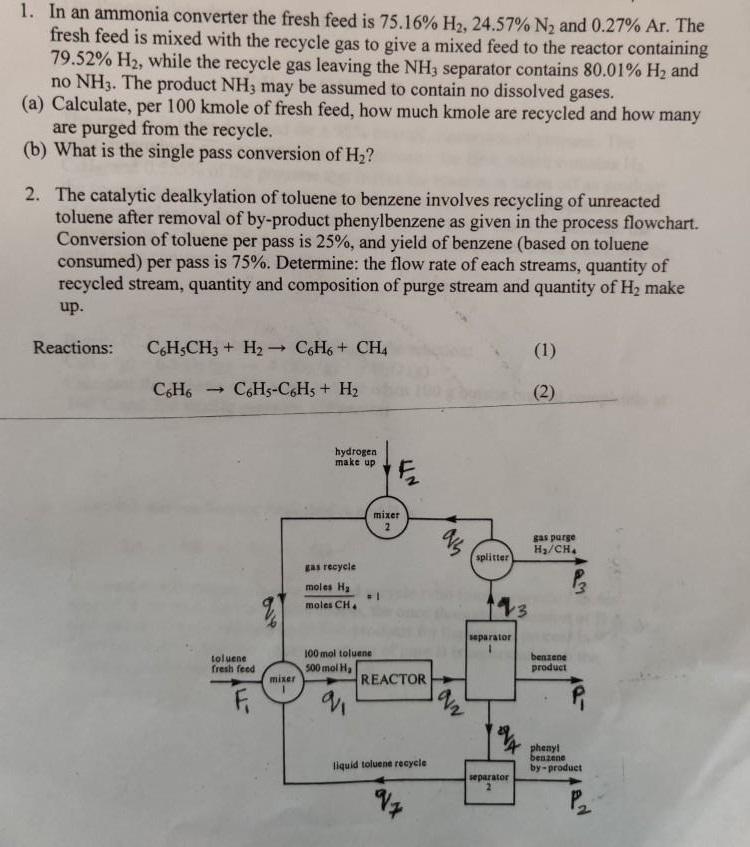
1. In an ammonia converter the fresh feed is 75.16% H, 24.57% N and 0.27% Ar. The fresh feed is mixed with the recycle gas to give a mixed feed to the reactor containing 79.52% H, while the recycle gas leaving the NH3 separator contains 80.01% H and no NH3. The product NH3 may be assumed to contain no dissolved gases. (a) Calculate, per 100 kmole of fresh feed, how much kmole are recycled and how many are purged from the recycle. (b) What is the single pass conversion of H? 2. The catalytic dealkylation of toluene to benzene involves recycling of unreacted toluene after removal of by-product phenylbenzene as given in the process flowchart. Conversion of toluene per pass is 25%, and yield of benzene (based on toluene consumed) per pass is 75%. Determine: the flow rate of each streams, quantity of recycled stream, quantity and composition of purge stream and quantity of H make up. Reactions: C6H5CH3 + H- C6H6+ CH4 C6H5-C6H5 + H C6H6 1 toluene fresh feed mixer hydrogen make up gas recycle moles H moles CH 100 mol toluene 500 mol H NTT mixer 2 REACTOR liquid toluene recycle 95 AN splitter 13 separator separator (1) (2) gas purge H/CH. benzene product phenyl benzene by-product
Step by Step Solution
★★★★★
3.42 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Question 1 Solution 1 Question 2 Solution 2 F 751644 79521H 2457Y N 027 Aj Reactor Bas...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635e338e064fa_182310.pdf
180 KBs PDF File
635e338e064fa_182310.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


