Answered step by step
Verified Expert Solution
Question
1 Approved Answer
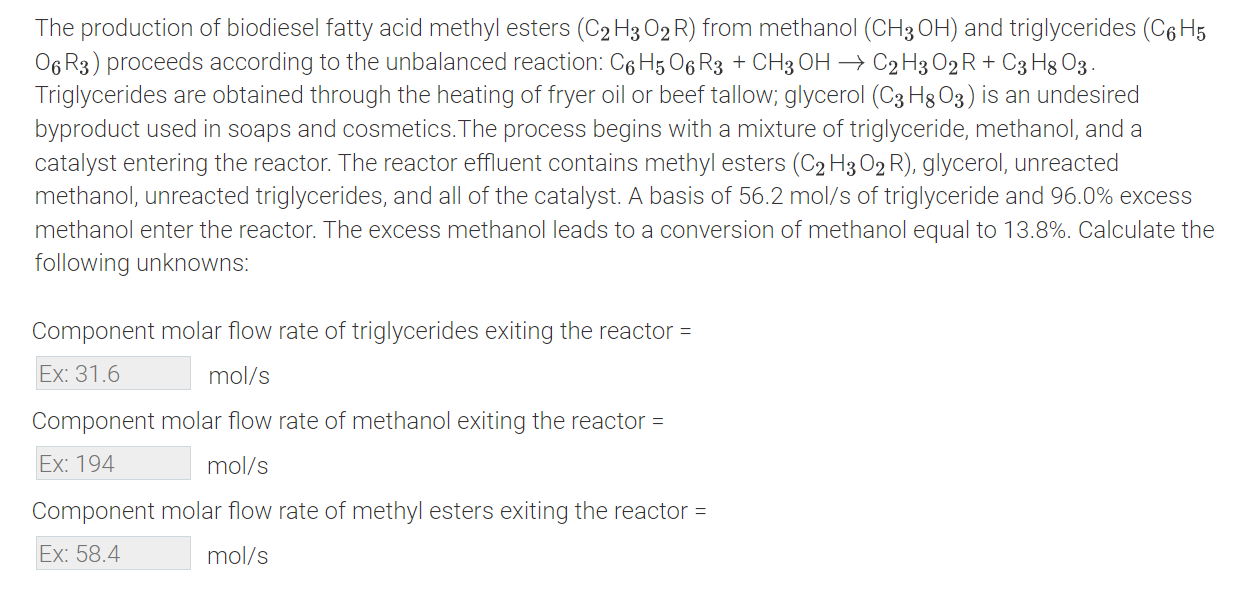
The production of biodiesel fatty acid methyl esters ( C 2 H 3 O 2 R ) from methanol ( C H 3 O H
The production of biodiesel fatty acid methyl esters from methanol and triglycerides
proceeds according to the unbalanced reaction:
Triglycerides are obtained through the heating of fryer oil or beef tallow; glycerol is an undesired
byproduct used in soaps and cosmetics The process begins with a mixture of triglyceride, methanol, and a
catalyst entering the reactor. The reactor effluent contains methyl esters glycerol, unreacted
methanol, unreacted triglycerides, and all of the catalyst. A basis of of triglyceride and excess
methanol enter the reactor. The excess methanol leads to a conversion of methanol equal to Calculate the
following unknowns:
Component molar flow rate of triglycerides exiting the reactor
Component molar flow rate of methanol exiting the reactor
Component molar flow rate of methyl esters exiting the reactor
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


