Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The pyrite rock is grinded into fine powder using mills and is then sent to the furnace (Stream F) where all sulphur in the ore
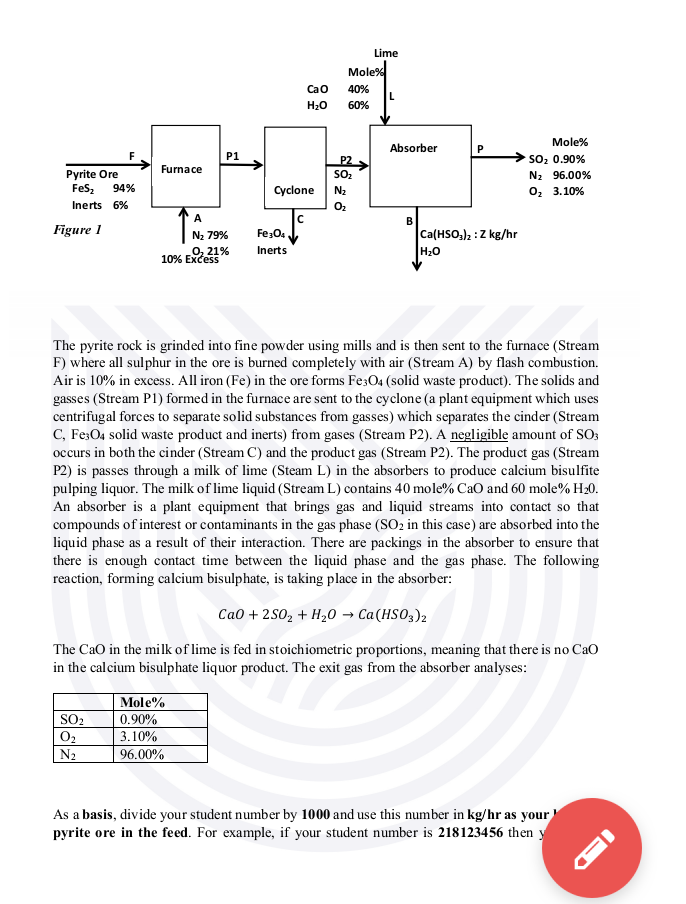

The pyrite rock is grinded into fine powder using mills and is then sent to the furnace (Stream F) where all sulphur in the ore is burned completely with air (Stream A) by flash combustion. Air is 10% in excess. All iron ( Fe ) in the ore forms Fe3O4 (solid waste product). The solids and gasses (Stream P1) formed in the furnace are sent to the cyclone (a plant equipment which uses centrifugal forces to separate solid substances from gasses) which separates the cinder (Stream C,Fe3O4 solid waste product and inerts) from gases (Stream P2). A negligible amount of SO3 occurs in both the cinder (Stream C) and the product gas (Stream P2). The product gas (Stream P2 ) is passes through a milk of lime (Steam L) in the absorbers to produce calcium bisulfite pulping liquor. The milk of lime liquid (Stream L) contains 40 mole %CaO and 60 mole %H20. An absorber is a plant equipment that brings gas and liquid streams into contact so that compounds of interest or contaminants in the gas phase ( SO2 in this case) are absorbed into the liquid phase as a result of their interaction. There are packings in the absorber to ensure that there is enough contact time between the liquid phase and the gas phase. The following reaction, forming calcium bisulphate, is taking place in the absorber: CaO+2SO2+H2OCa(HSO3)2 The CaO in the milk of lime is fed in stoichiometric proportions, meaning that there is no CaO in the calcium bisulphate liquor product. The exit gas from the absorber analyses: As a basis, divide your student number by 1000 and use this number in kg/hr as yo pyrite ore in the feed. For example, if your student number is 218123456 then The pyrite rock is grinded into fine powder using mills and is then sent to the furnace (Stream F) where all sulphur in the ore is burned completely with air (Stream A) by flash combustion. Air is 10% in excess. All iron ( Fe ) in the ore forms Fe3O4 (solid waste product). The solids and gasses (Stream P1) formed in the furnace are sent to the cyclone (a plant equipment which uses centrifugal forces to separate solid substances from gasses) which separates the cinder (Stream C,Fe3O4 solid waste product and inerts) from gases (Stream P2). A negligible amount of SO3 occurs in both the cinder (Stream C) and the product gas (Stream P2). The product gas (Stream P2) is passes through a milk of lime (Steam L) in the absorbers to produce calcium bisulfite pulping liquor. The milk of lime liquid (Stream L) contains 40 mole %CaO and 60mole%H20. An absorber is a plant equipment that brings gas and liquid streams into contact so that compounds of interest or contaminants in the gas phase ( SO2 in this case) are absorbed into the liquid phase as a result of their interaction. There are packings in the absorber to ensure that there is enough contact time between the liquid phase and the gas phase. The following reaction, forming calcium bisulphate, is taking place in the absorber: CaO+2SO2+H2OCa(HSO3)2 The CaO in the milk of lime is fed in stoichiometric proportions, meaning that there is no CaO in the calcium bisulphate liquor product. The exit gas from the absorber analyses: As a basis, divide your student number by 1000 and use this number in kg/hr as your basis of pyrite ore in the feed. For example, if your student number is 218123456 then your basis would be 218123456/1000=218123.46kg/hr of iron ore ( FeS2+ Inerts) in the feed. Note that this is compulsory, and any other standard assumptions of a basis are not allowed. You can use other sources of literature to assist you with solving the problem. Round off all your answers to 2 decimals. Use the following molecular weights for your calculations: Your Line Manager, Nathi Khoza, requested that you do the following: a) Conduct mass balance calculations for this new plant to determine Stream A, C, B, L, and P in kg/hr; and Z in kg/hr. Your calculations should be typed and layed out in an easy-to-understand manner. Typing of calculations and report is a must, handwritten reports will not be marked and automatically get a zero. (50 marks) The pyrite rock is grinded into fine powder using mills and is then sent to the furnace (Stream F) where all sulphur in the ore is burned completely with air (Stream A) by flash combustion. Air is 10% in excess. All iron ( Fe ) in the ore forms Fe3O4 (solid waste product). The solids and gasses (Stream P1) formed in the furnace are sent to the cyclone (a plant equipment which uses centrifugal forces to separate solid substances from gasses) which separates the cinder (Stream C,Fe3O4 solid waste product and inerts) from gases (Stream P2). A negligible amount of SO3 occurs in both the cinder (Stream C) and the product gas (Stream P2). The product gas (Stream P2 ) is passes through a milk of lime (Steam L) in the absorbers to produce calcium bisulfite pulping liquor. The milk of lime liquid (Stream L) contains 40 mole %CaO and 60 mole %H20. An absorber is a plant equipment that brings gas and liquid streams into contact so that compounds of interest or contaminants in the gas phase ( SO2 in this case) are absorbed into the liquid phase as a result of their interaction. There are packings in the absorber to ensure that there is enough contact time between the liquid phase and the gas phase. The following reaction, forming calcium bisulphate, is taking place in the absorber: CaO+2SO2+H2OCa(HSO3)2 The CaO in the milk of lime is fed in stoichiometric proportions, meaning that there is no CaO in the calcium bisulphate liquor product. The exit gas from the absorber analyses: As a basis, divide your student number by 1000 and use this number in kg/hr as yo pyrite ore in the feed. For example, if your student number is 218123456 then The pyrite rock is grinded into fine powder using mills and is then sent to the furnace (Stream F) where all sulphur in the ore is burned completely with air (Stream A) by flash combustion. Air is 10% in excess. All iron ( Fe ) in the ore forms Fe3O4 (solid waste product). The solids and gasses (Stream P1) formed in the furnace are sent to the cyclone (a plant equipment which uses centrifugal forces to separate solid substances from gasses) which separates the cinder (Stream C,Fe3O4 solid waste product and inerts) from gases (Stream P2). A negligible amount of SO3 occurs in both the cinder (Stream C) and the product gas (Stream P2). The product gas (Stream P2) is passes through a milk of lime (Steam L) in the absorbers to produce calcium bisulfite pulping liquor. The milk of lime liquid (Stream L) contains 40 mole %CaO and 60mole%H20. An absorber is a plant equipment that brings gas and liquid streams into contact so that compounds of interest or contaminants in the gas phase ( SO2 in this case) are absorbed into the liquid phase as a result of their interaction. There are packings in the absorber to ensure that there is enough contact time between the liquid phase and the gas phase. The following reaction, forming calcium bisulphate, is taking place in the absorber: CaO+2SO2+H2OCa(HSO3)2 The CaO in the milk of lime is fed in stoichiometric proportions, meaning that there is no CaO in the calcium bisulphate liquor product. The exit gas from the absorber analyses: As a basis, divide your student number by 1000 and use this number in kg/hr as your basis of pyrite ore in the feed. For example, if your student number is 218123456 then your basis would be 218123456/1000=218123.46kg/hr of iron ore ( FeS2+ Inerts) in the feed. Note that this is compulsory, and any other standard assumptions of a basis are not allowed. You can use other sources of literature to assist you with solving the problem. Round off all your answers to 2 decimals. Use the following molecular weights for your calculations: Your Line Manager, Nathi Khoza, requested that you do the following: a) Conduct mass balance calculations for this new plant to determine Stream A, C, B, L, and P in kg/hr; and Z in kg/hr. Your calculations should be typed and layed out in an easy-to-understand manner. Typing of calculations and report is a must, handwritten reports will not be marked and automatically get a zero. (50 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


