Question
The qualitative sketches I, II and III given below show the variation of surface tension with a molar concentration of three different aqueous solutions of
The qualitative sketches I, II and III given below show the variation of surface tension with a molar concentration of three different aqueous solutions of KCl, CH3OH and CH3(CH2)111OSO3?Na+ at room temperature.
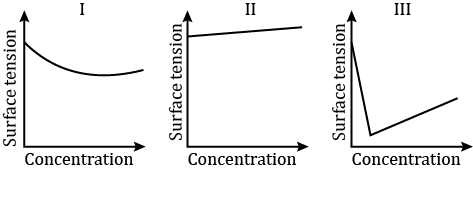
The correct assignment of the sketches is:
(A) I:KCl; II:CH3OH; III:CH3(CH2)11OSO3? Na+
(B) I:CH3(CH2)11OSO3? Na+: II:CH3OH; III:KCl
(C) I:KCl; II:CH3(CH2)11OSO3? Na+; III:CH3OH
(D) I:CH3OH; II:KCl; III:CH3(CH2)11OSO3? Na+
Surface tension I Concentration Surface tension II Concentration Surface tension III Concentration
Step by Step Solution
3.47 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



