Question
The rate constant of an ionic reaction depends also upon the ionic strength of the solution known as primary kinetic salt effect What are
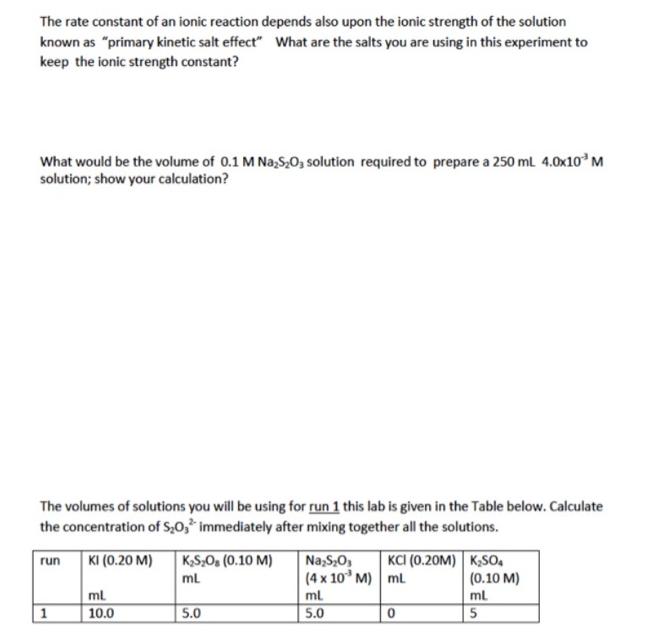
The rate constant of an ionic reaction depends also upon the ionic strength of the solution known as "primary kinetic salt effect" What are the salts you are using in this experiment to keep the ionic strength constant? What would be the volume of 0.1 M NaSO3 solution required to prepare a 250 mL 4.0x10 M solution; show your calculation? The volumes of solutions you will be using for run 1 this lab is given in the Table below. Calculate the concentration of S0 immediately after mixing together all the solutions. run 1 KI (0.20 M) KSO (0.10 M) mL ml. 10.0 5.0 NaSO, (4 x 10 M) ml. 5.0 KCI (0.20M) KSO4 mL 0 (0.10 M) ml 5
Step by Step Solution
3.53 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
1 Primary Kinetic Salt Effect The primary kinetic salt effect refers to how the rate constant of an ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Smith and Roberson Business Law
Authors: Richard A. Mann, Barry S. Roberts
15th Edition
1285141903, 1285141903, 9781285141909, 978-0538473637
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



