Question
The rate data in Table 1 were obtained for the reaction of A+ S --> U. What is the order of the reaction with
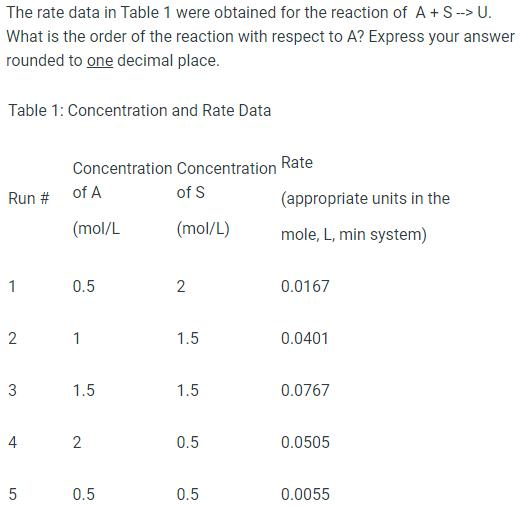
The rate data in Table 1 were obtained for the reaction of A+ S --> U. What is the order of the reaction with respect to A? Express your answer rounded to one decimal place. Table 1: Concentration and Rate Data Run # 1 2 3 4 5 Concentration of A (mol/L 0.5 1 1.5 2 0.5 Concentration of S (mol/L) 2 1.5 1.5 0.5 0.5 Rate (appropriate units in the mole, L, min system) 0.0167 0.0401 0.0767 0.0505 0.0055
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To determine the order of the reaction with respect to reactant A we need to look at how changes in ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Design And Analysis Of Experiments
Authors: Douglas C., Montgomery
5th Edition
978-0471316497, 0471316490
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



