Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The rate of effusion of unknown gas was measured and found to be 34.9mL/min. Under the same conditions, the rate of effusion of O,

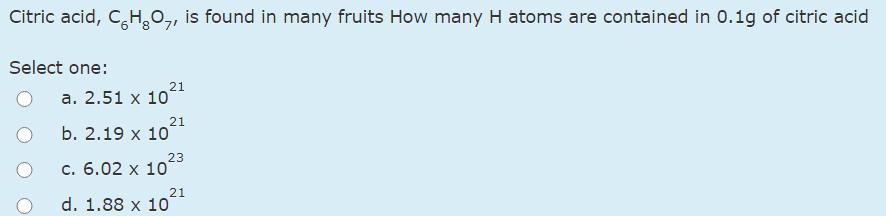

The rate of effusion of unknown gas was measured and found to be 34.9mL/min. Under the same conditions, the rate of effusion of O, was found to be 24.6mL/min. Calculate the molar mass of the unknown gas (in g/mol). Select one: . 54.2 b. 15.9 . 37.9 d. 43.9 In the following reaction, what ions, if any, are spectators ions? AGNO, + KI AgI + 2KNO, Select one: + . NO 3(aq) (aq)' b. Ag NO (aq)' 3(aq) c. Ag (aq)' (aq) I d. K Ag (aq)' (aq) . I (aq)' (aq)
Step by Step Solution
★★★★★
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


