Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The rate of polymerization in an acid-catalyzed polyesterification is given by -d[OH] -d[COOH] = k* [COOH ] [OH] dt dt The rate of polymerization in
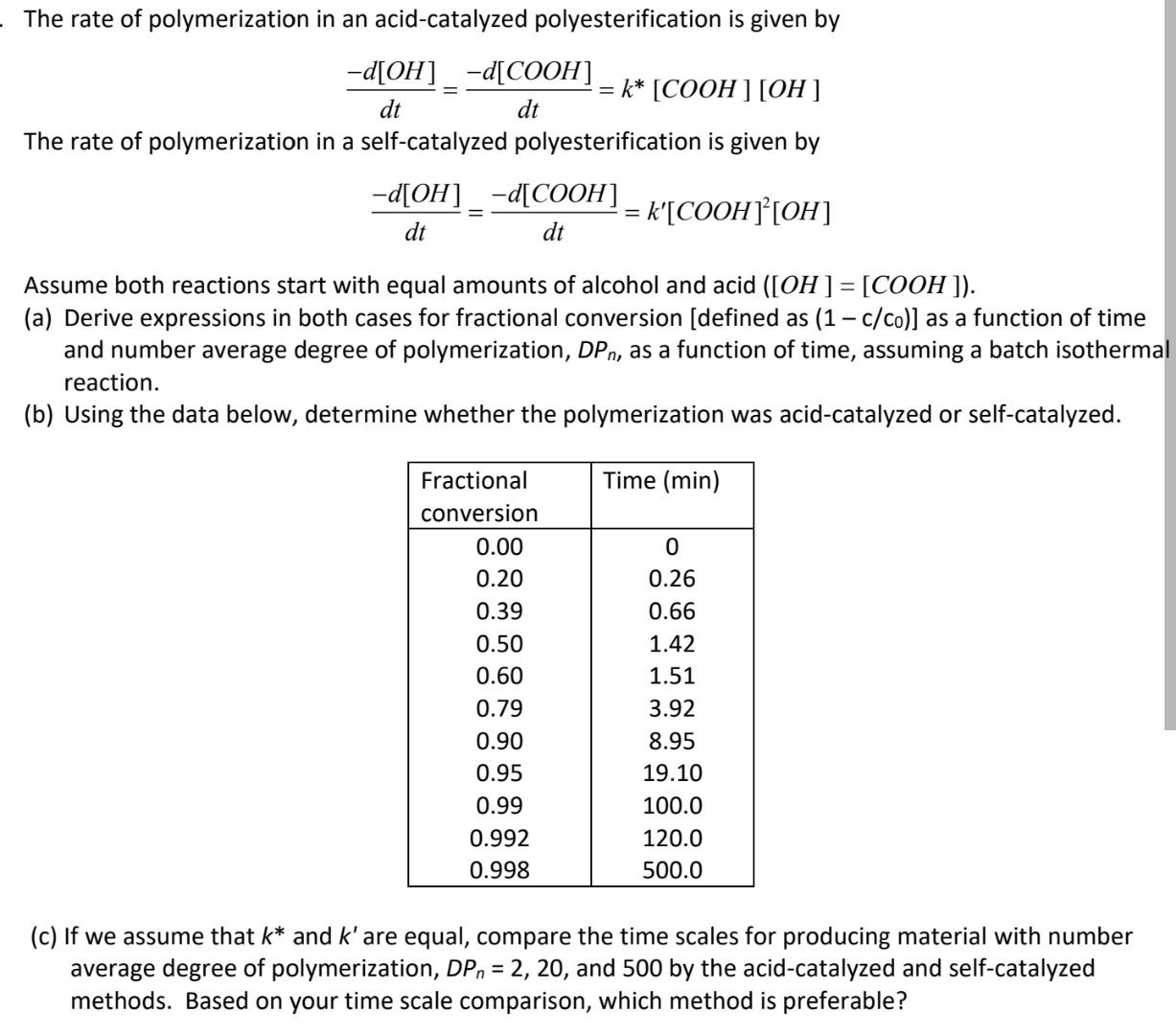
The rate of polymerization in an acid-catalyzed polyesterification is given by -d[OH] -d[COOH] = k* [COOH ] [OH] dt dt The rate of polymerization in a self-catalyzed polyesterification is given by -d[OH]_-d[COOH] = k'[COOH] [OH] dt dt Assume both reactions start with equal amounts of alcohol and acid ([OH ] = [COOH ]). (a) Derive expressions in both cases for fractional conversion (defined as (1 - c/co)] as a function of time and number average degree of polymerization, DPn, as a function of time, assuming a batch isothermal reaction. (b) Using the data below, determine whether the polymerization was acid-catalyzed or self-catalyzed. , Time (min) Fractional conversion 0.00 0.20 0.39 0.50 0.60 0.79 0.90 0.95 0.99 0.992 0.998 0 0.26 0.66 1.42 1.51 3.92 8.95 19.10 100.0 120.0 500.0 (c) If we assume that k* and k' are equal, compare the time scales for producing material with number average degree of polymerization, DP, = 2, 20, and 500 by the acid-catalyzed and self-catalyzed methods. Based on your time scale comparison, which method is preferable
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


