Answered step by step
Verified Expert Solution
Question
1 Approved Answer
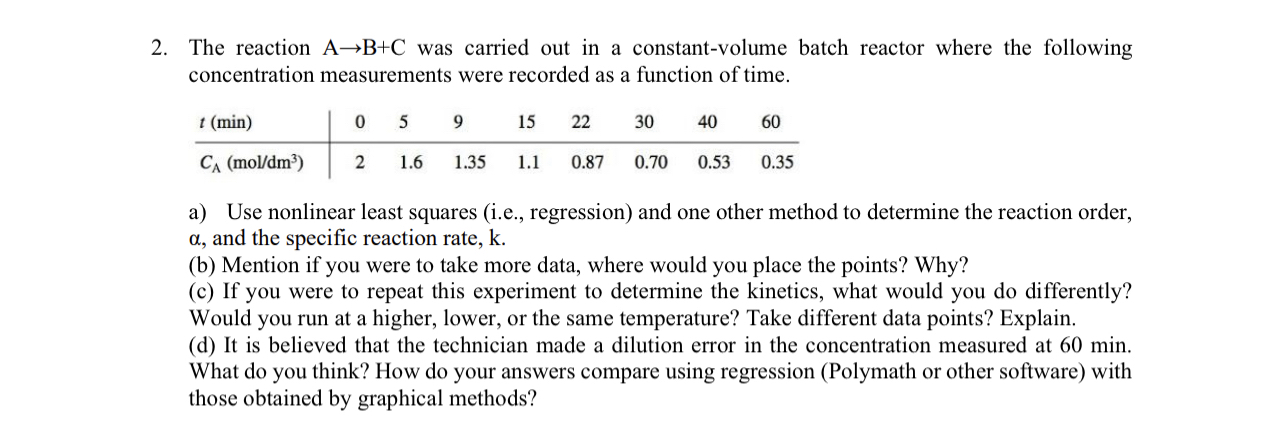
The reaction A B + C was carried out in a constant - volume batch reactor where the following concentration measurements were recorded as a
The reaction was carried out in a constantvolume batch reactor where the following concentration measurements were recorded as a function of time.
table
a Use nonlinear least squares ie regression and one other method to determine the reaction order, and the specific reaction rate,
b Mention if you were to take more data, where would you place the points? Why?
c If you were to repeat this experiment to determine the kinetics, what would you do differently? Would you run at a higher, lower, or the same temperature? Take different data points? Explain.
d It is believed that the technician made a dilution error in the concentration measured at min. What do you think? How do your answers compare using regression Polymath or other software with those obtained by graphical methods?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


