Question
The reaction A(aq) + B(aq) Products(aq) was studied, and the following data were obtained: [A]o (mol/L) 0.24 0.12 0.060 0.48 0.36 Submit 0.24 0.18
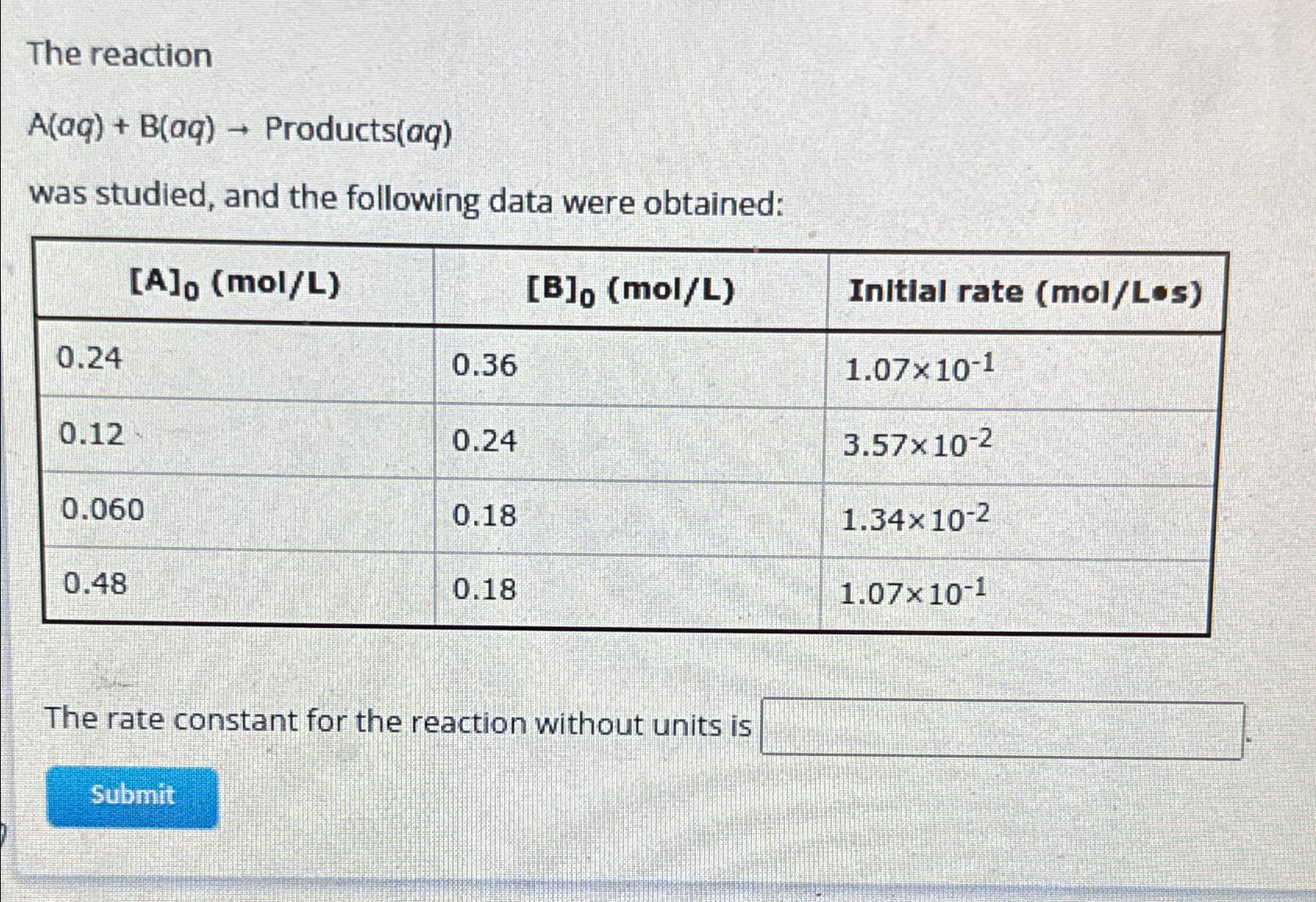
The reaction A(aq) + B(aq) Products(aq) was studied, and the following data were obtained: [A]o (mol/L) 0.24 0.12 0.060 0.48 0.36 Submit 0.24 0.18 0.18 [B], (mol/L) The rate constant for the reaction without units is Initial rate (mol/Los) 1.0710-1 3.57x10-2 1.34x10-2 1.07x10-1
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Given is a general reaction of two reactants A and B forming products We will fin...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
8th Edition
1305581989, 978-1305581982
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



