Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The reaction mechanism for the decomposition of A 2 is attached. where the dissociation of A 2 is first order in A 2 , and
The reaction mechanism for the decomposition of A2 is attached. where the dissociation of A2 is first order in A2, and the recombination of A is second order in A; the reaction of A and B is first order in both A and B. Deduce the rate law for the rate of formation of P in two ways: (i) assuming a pre-equilibrium between A2 and A and (ii) applying the steady state approximation to A.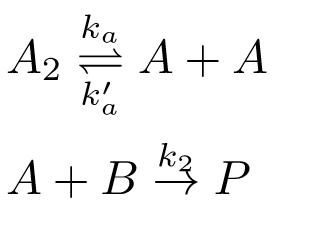
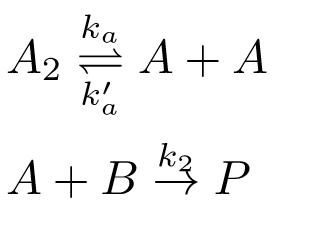
ka A2 = A+ A k'a + %
Step by Step Solution
★★★★★
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636107e2182cf_235281.pdf
180 KBs PDF File
636107e2182cf_235281.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


