Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The reaction of RMgX with the Formaldehyde give Select one: a. Ketone b. Aldehyde c. alcohol d. Halide Alkyl within the following reaction: Mg+R2HgHg+R2Mg If
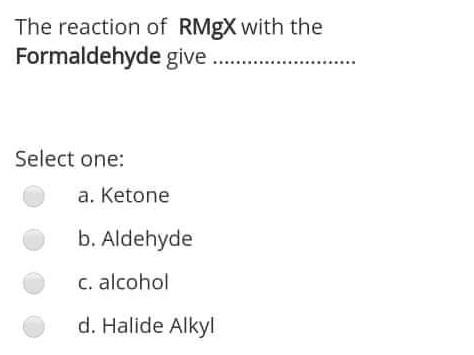


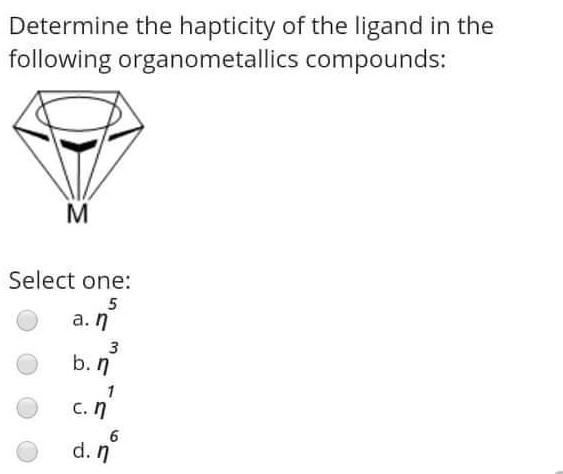
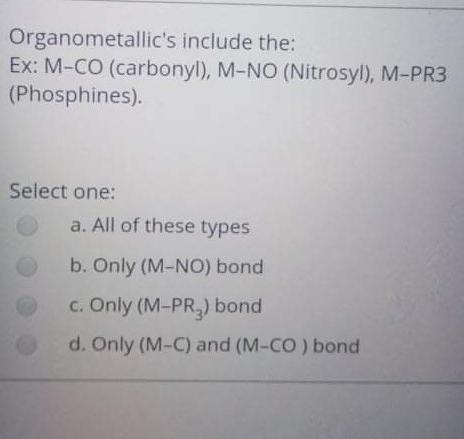


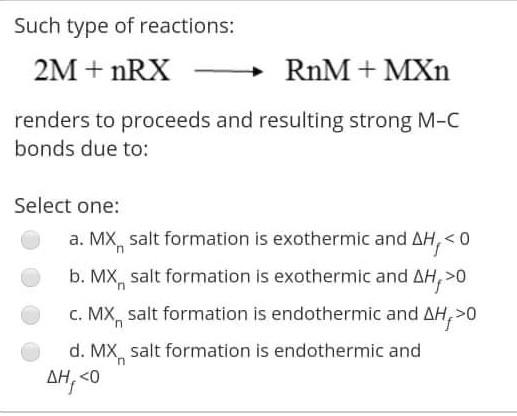










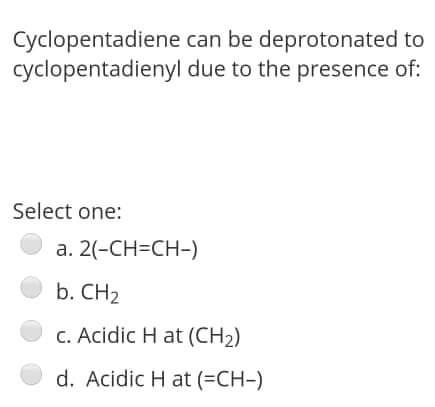
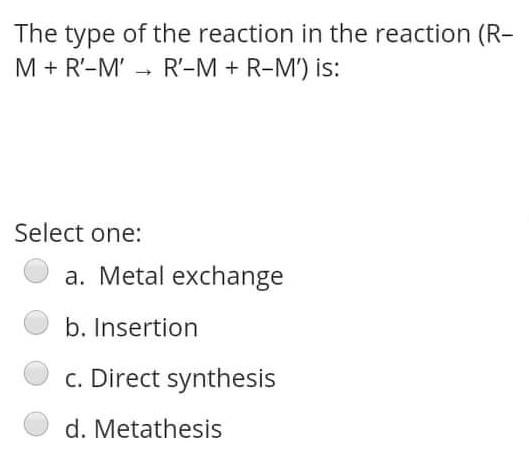

The reaction of RMgX with the Formaldehyde give Select one: a. Ketone b. Aldehyde c. alcohol d. Halide Alkyl within the following reaction: Mg+R2HgHg+R2Mg If the R is [C(SiMe3)3], then the R2Mg in solid state will be found as: Select one: a. Monemer b. Dimer c. reaction can't proceed! d. Trimer The reaction of RMgX with the Esters give Select one: a. Tertiary Alcohol b. Primary alcohol c. Secondary Alcohol d. All of them Determine the hapticity of the ligand in the following organometallics compounds: Select one: a. 5 b. 3 c. 1 d. 6 Organometallic's include the: Ex: M-CO (carbonyl), M-NO (Nitrosyl), M-PR3 (Phosphines). Select one: a. All of these types b. Only (M-NO) bond c. Only (M-PR R3 ) bond d. Only (M-C) and (M-CO) bond Tetraphenylborate ion ([BPh4] is used to: Select one: a. Solubilize large metal ions b. Precipitate large metal anions c. Enhance reactivity of ions d. Precipitate large metal cations Tetraphenylborate ion ([BPh ]4] is used to: Select one: a. Precipitate large metal anions b. Solubilize large metal ions c. Enhance reactivity of ions d. Precipitate large metal cations Such type of reactions: 2M+nRXRnM+MXn renders to proceeds and resulting strong M-C bonds due to: Select one: a. MXn salt formation is exothermic and Hf0 c. MXn salt formation is endothermic and Hf>0 d. MXn salt formation is endothermic and Hf
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


