Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The real gas behavior of ethanol can be expressed by using the following Virial equation model for the compressibility factor: Z = 1 + (BP/RT),
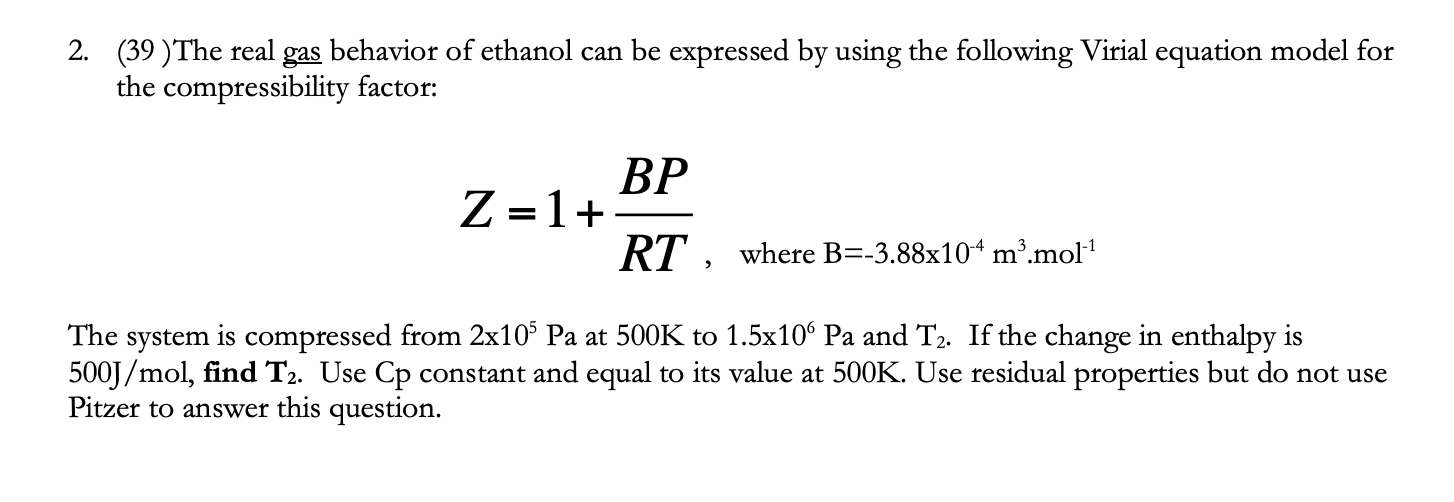 The real gas behavior of ethanol can be expressed by using the following Virial equation model for the compressibility factor: Z = 1 + (BP/RT), where B=-3.88x10^-4 m3/mol The system is compressed from 2x10^5 Pa at 500K to 1.5x10^6 Pa and T2. If the change in enthalpy is 500J/mol, find T2. Use Cp constant and equal to its value at 500K. Use residual properties but do not use Pitzer to answer this question.
The real gas behavior of ethanol can be expressed by using the following Virial equation model for the compressibility factor: Z = 1 + (BP/RT), where B=-3.88x10^-4 m3/mol The system is compressed from 2x10^5 Pa at 500K to 1.5x10^6 Pa and T2. If the change in enthalpy is 500J/mol, find T2. Use Cp constant and equal to its value at 500K. Use residual properties but do not use Pitzer to answer this question.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


