Question
In general, it is possible to determine if large molecules, such as Vitamin D and Vitamin C, are considered polar or nonpolar by examining
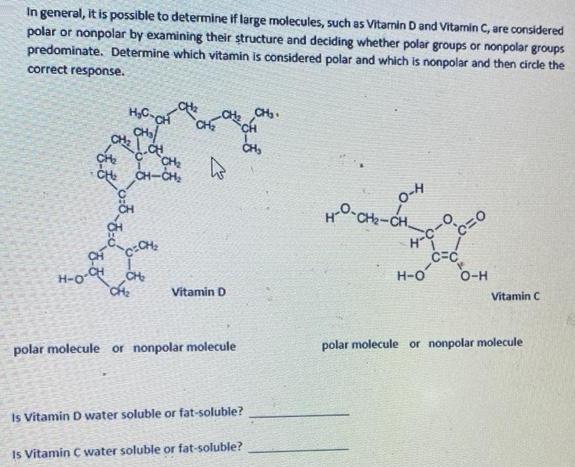
In general, it is possible to determine if large molecules, such as Vitamin D and Vitamin C, are considered polar or nonpolar by examining their structure and deciding whether polar groups or nonpolar groups predominate. Determine which vitamin is considered polar and which is nonpolar and then circle the correct response. HC-CH CH0 CH CH CH CH CH-CH CH C=CH H-O-OH CH k Vitamin D polar molecule or nonpolar molecule Is Vitamin D water soluble or fat-soluble? Is Vitamin C water soluble or fat-soluble? CH. 0-4 HO CHCH -C- H-9 C=C H-O c=0 O-H Vitamin C polar molecule or nonpolar molecule
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
structures H0 1 CH CH CH CH CH CH C CH3 CH3 CH CH2 of vitamin D CH CM2 CH CHCH a CH CH vitami...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
8th Extended edition
471758019, 978-0471758013
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



