Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Redlich-Kwong equation of state is given by RT a P = (V - b) v(V + b)VT where a = 0.42747 and b
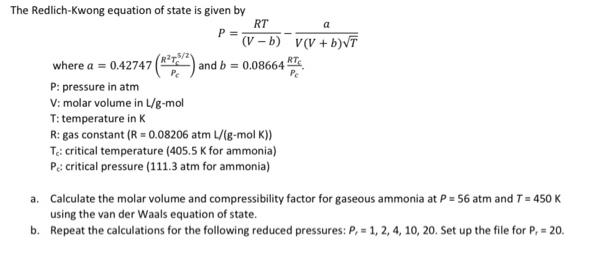
The Redlich-Kwong equation of state is given by RT a P = (V - b) v(V + b)VT where a = 0.42747 and b = 0.08664 P: pressure in atm V: molar volume in /g-mol T: temperature in K R: gas constant (R = 0.08206 atm L/(g-mol K)) Te: critical temperature (405.5 K for ammonia) P critical pressure (111.3 atm for ammonia) a. Calculate the molar volume and compressibility factor for gaseous ammonia at P = 56 atm and T= 450 K using the van der Waals equation of state. b. Repeat the calculations for the following reduced pressures: P, = 1, 2, 4, 10, 20. Set up the file for P, = 20.
Step by Step Solution
★★★★★
3.51 Rating (175 Votes )
There are 3 Steps involved in it
Step: 1
Question 1 Calculate the molar volume and compressibility factor for gaseous ammonia at P 56 atm and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


