Question
The removal of benzene by holding it on Activated Carbon can be expressed by the Freundlich isotherm given below. Here, c (mg/L) and q (mg/g)
The removal of benzene by holding it on Activated Carbon can be expressed by the Freundlich isotherm given below.
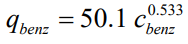
Here, c (mg/L) and q (mg/g) show the concentration of benzene and the capacity of activated carbon, respectively. A solution with 0.50 mg/l benzene at a temperature of 25 oC will be treated using a batch reactor. 0.01 mg/l benzene is required in the water coming out of the treatment. Calculate the amount of activated carbon required, according to the fact that the specific surface area of Activated Carbon to be used in the batch reactor is known to be 650 m2/g.
9 benz 0.533 benz = 50.1 c
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics For Engineers And Scientists
Authors: William Navidi
3rd Edition
73376345, 978-0077417581, 77417585, 73376337, 978-0073376332
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



