Answered step by step
Verified Expert Solution
Question
1 Approved Answer
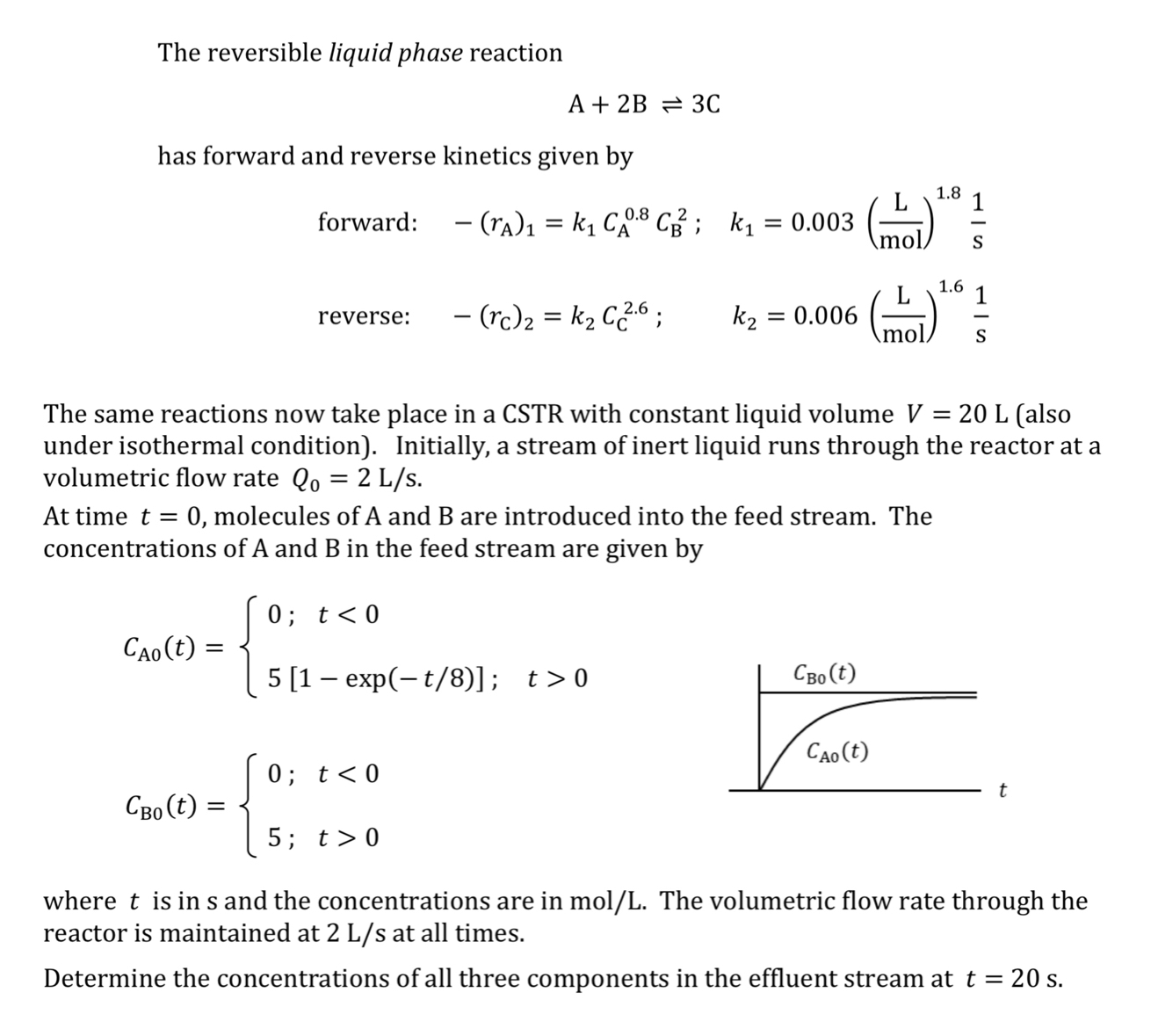
The reversible liquid phase reaction A + 2 B 3 C has forward and reverse kinetics given by forward: - ( r A ) 1
The reversible liquid phase reaction
has forward and reverse kinetics given by
forward: ;
reverse: ;
The same reactions now take place in a CSTR with constant liquid volume also under isothermal condition Initially, a stream of inert liquid runs through the reactor at a volumetric flow rate
At time molecules of A and B are introduced into the feed stream. The concentrations of A and in the feed stream are given by
exp;
;
where is in s and the concentrations are in molL The volumetric flow rate through the reactor is maintained at at all times.
Determine the concentrations of all three components in the effluent stream at
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


