The reversible set of reactions represented by ki ka AB20 is carried out in a batch reactor under conditions of constant volume and temperature.
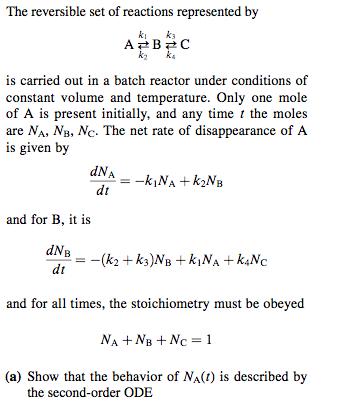
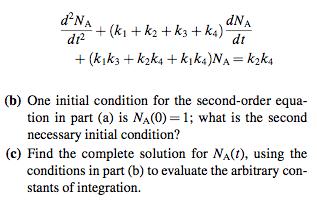
The reversible set of reactions represented by ki ka AB20 is carried out in a batch reactor under conditions of constant volume and temperature. Only one mole of A is present initially, and any time t the moles are NA, NB, Nc. The net rate of disappearance of A is given by dNA -k|NA + K2NB dt and for B, it is dNB dt -(k2 + k3)NB + kjNA + kNC and for all times, the stoichiometry must be obeyed NA + NB + Nc = 1 (a) Show that the behavior of NA(t) is described by the second-order ODE d'NA + (k + k2 + k3 + ka) dNA dr? dt + (kk3 + kzka + kka)NA = kaka (b) One initial condition for the second-order equa- tion in part (a) is NA(0) = 1; what is the second necessary initial condition? (c) Find the complete solution for NA(1), using the conditions in part (b) to evaluate the arbitrary con- stants of integration.
Step by Step Solution
3.56 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


