Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The scientists, discouraged by the breakdown of the newly discovered gas, are not disappointed for long. They have found that a product of CD3, gaseous
The scientists, discouraged by the breakdown of the newly discovered gas, are not disappointed for long. They have found that a product of CD3, gaseous D2, has many practical uses! Given a sample of CD3 in a fixed-volume 2.0 L container at 54.0 C with an initial total pressure of 4.0 bar, calculate the theoretical yield, in mol, of D2. (R = 0.08314 L bar mol-1 K-1)
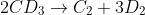
| Time (sec) | PC2 (bar) | PD2 (bar) |
| 0 | 0 | 0 |
| 60 | 0.5 | N/A |
| 120 | 1.0 | N/A |
| 180 | 1.5 | N/A |
| 240 | 2.0 | N/A |
Your Answer:
|
| |
| Answer | units |
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


