Answered step by step
Verified Expert Solution
Question
1 Approved Answer
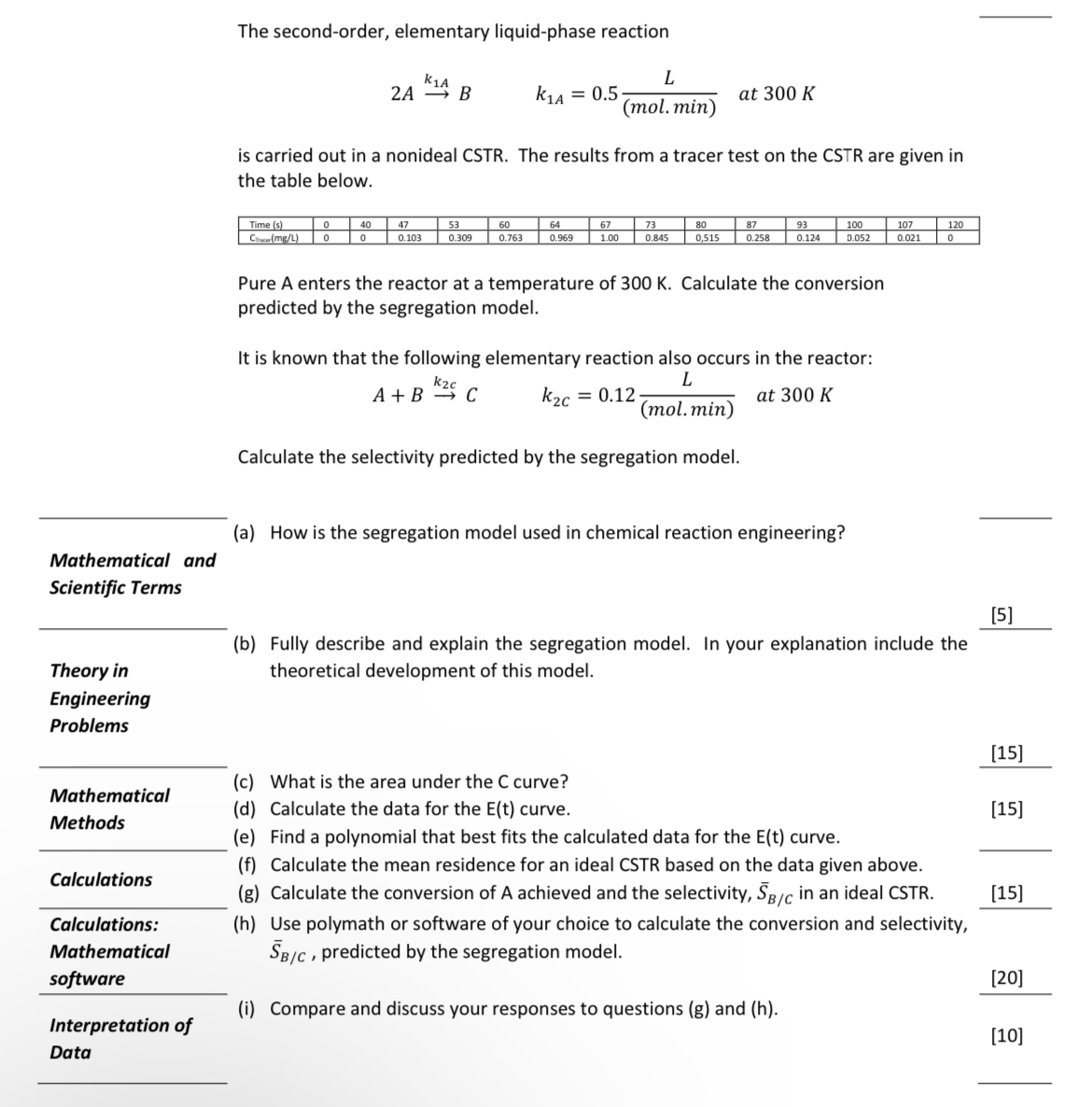
The second - order, elementary liquid - phase reaction 2 A k 1 A B , k 1 A = 0 . 5 L (
The secondorder, elementary liquidphase reaction
is carried out in a nonideal CSTR The results from a tracer test on the CSTR are given in the table below.
tableTime sCiran
Pure A enters the reactor at a temperature of Calculate the conversion predicted by the segregation model.
It is known that the following elementary reaction also occurs in the reactor:
Calculate the selectivity predicted by the segregation model.
a How is the segregation model used in chemical reaction engineering?
Mathematical and
Scientific Terms
b Fully describe and explain the segregation model. In your explanation include the
Theory in theoretical development of this model.
Engineering
Problems
Mathematical
Methods
Calculations
Calculations:
Mathematical
software
Interpretation of
Data
c What is the area under the C curve?
d Calculate the data for the curve.
e Find a polynomial that best fits the calculated data for the curve.
f Calculate the mean residence for an ideal CSTR based on the data given above.
g Calculate the conversion of A achieved and the selectivity, in an ideal CSTR
h Use polymath or software of your choice to calculate the conversion and selectivity, predicted by the segregation model.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


