Question
The specific heat of a certain type of cooking oil is 1.75 J/(g.C). How much heat energy is needed to raise the temperature of


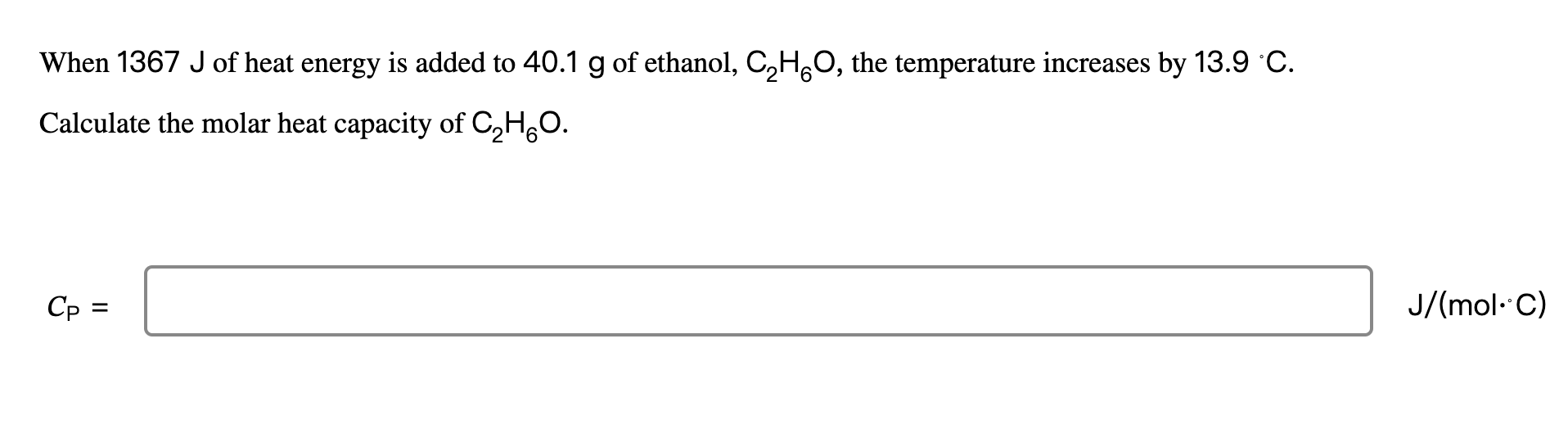
The specific heat of a certain type of cooking oil is 1.75 J/(g.C). How much heat energy is needed to raise the temperature of 2.92 kg of this oil from 23 C to 191 C? 9 = J Question 5 of 26 > An 80.0 g sample of a gas was heated from 25 C to 225 C. During this process, 346 J of work was done by the system and its internal energy increased by 7295 J. What is the specific heat of the gas? C = x10 TOOLS J/(g. C) When 1367 J of heat energy is added to 40.1 g of ethanol, C2H6O, the temperature increases by 13.9 C. Calculate the molar heat capacity of CHO. = J/(mol. C)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



